The 'Grid' field in EDC/CDMS
Table of Contents
Grid fields are used to group closely related data in a tight interface (table).
To create a grid field, in 'Study form' > 'Visits' select field type 'Grid' from the list of field types.
The 'Add a new field' dialog window will appear:
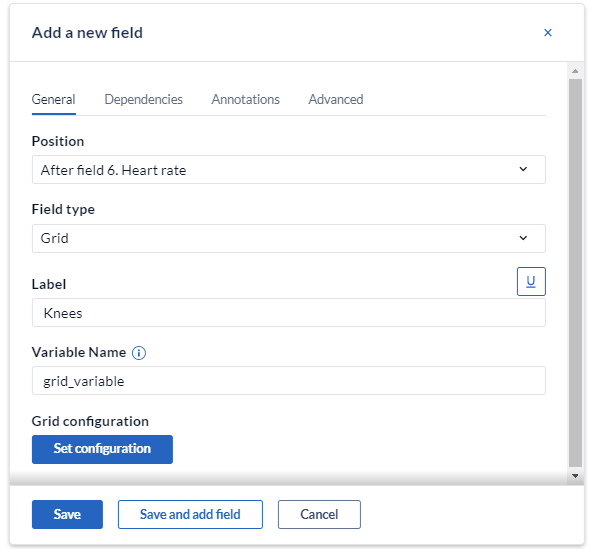
Choose the position, add a label and name the variable. Once completed, click 'Set configuration' to open the configuration dialog in which the rows and columns in your grid can be defined:
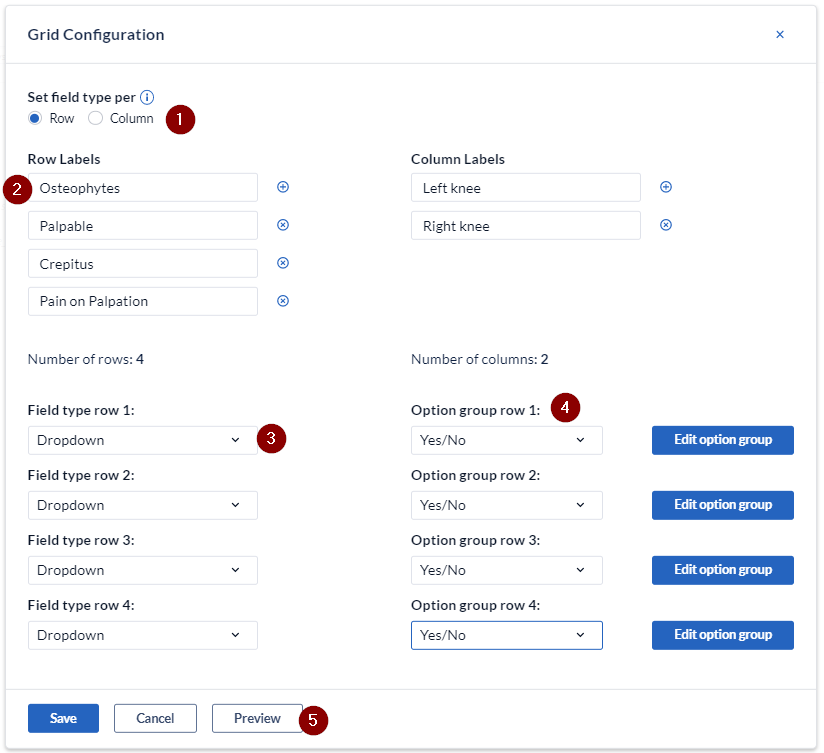
- Determine whether the field type is to be set by column (each column will have the same field type) or row (each row will have the same field type).
- Enter the labels for the rows and columns. Add or remove rows and columns by using the + / X signs.
- Set the field type per row - these can be set as text, number, dropdown or date fields.
- Set the option group for the associated field. Select from the existing groups or create a new one by selecting "New option group".
- You can check the grid field configuration by clicking the 'Preview' button.
Once all fields are complete, click the 'Save' button to save the configuration. The 'Grid configuration' window will close, click 'Save' in the 'Add new field' dialog to close the window and create the field.
Here is an example of a grid field as seen in the study forms:
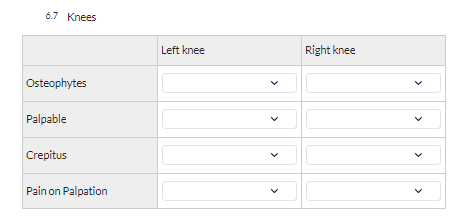
Note: After data has been collected in a grid field, it is not possible to change the number of rows or columns, nor the selected field types. If it is absolutely necessary to make changes to the configuration, we advise to copy the grid field, change the copied one and keep both instances for all participants.
Other grid field properties
It is not possible to:
- Add validations to grid fields
- Make a grid field required
- Mark grid field data as missing
- Adjust the column or row size
If you have too many rows or columns, the users will need to scroll left/right and up/down to be able to fill in the whole grid and might omit some fields. In this case, it is advised to consider using the Repeated Measure field instead. Grid fields have certain limitations and in some cases it is better to use Repeated measure repeating data structure. Learn more when to use grid fields or repeated measure in this article: 'Should I use a Grid or a Repeated measure in EDC/CDMS?'
- You can use the grid field in certain calculations. The gridfields are saved as so-called JSON objects and this is what you will need to define in the calculations.
- When a grid field is exported, each grid column is exported as its own column, which is labeled as Variablename_rowname_columnname