Archive or unarchive a participant / survey / repeating data in CDMS
Table of Contents
Once the study is live, it is no longer possible to delete a participant/survey/repeating data. The only way to remove it from the current overview is to archive it.
Archiving in Castor behaves almost in the same way as deleting - archived instances do not get exported with the data and do not influence the results of the study. The difference between deletion and archival is that you can always retrieve an archived instance by unarchiving it. This is necessary for GCP regulations that require full traceability of the study.
Archiving participants
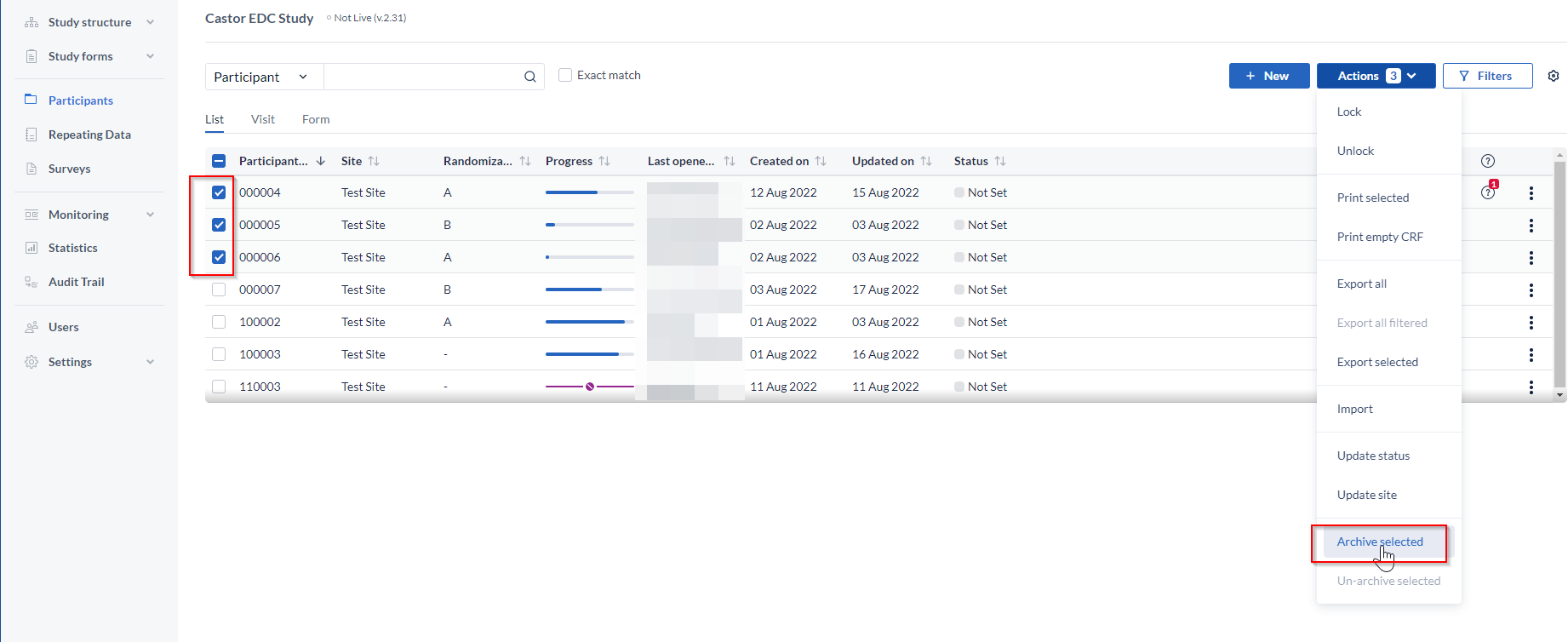
To archive - select a participant or several participants you would like to archive, click on the 'Actions' button and select the 'Archive selected' option, then enter the reason for archiving:
It will still be possible to access archived participants, surveys, or repeating data by checking the filter for Archived instances in the relevant overview.
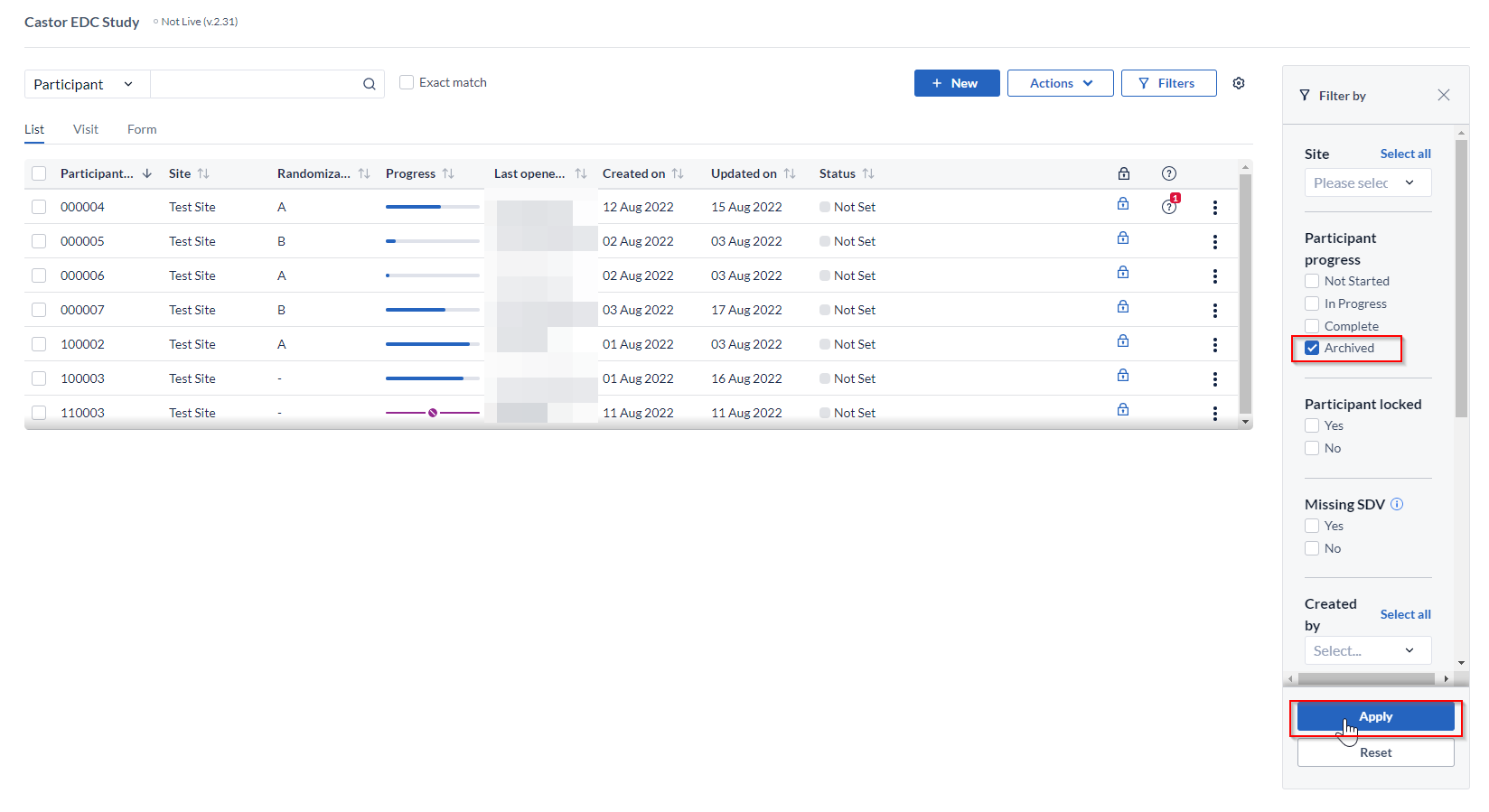
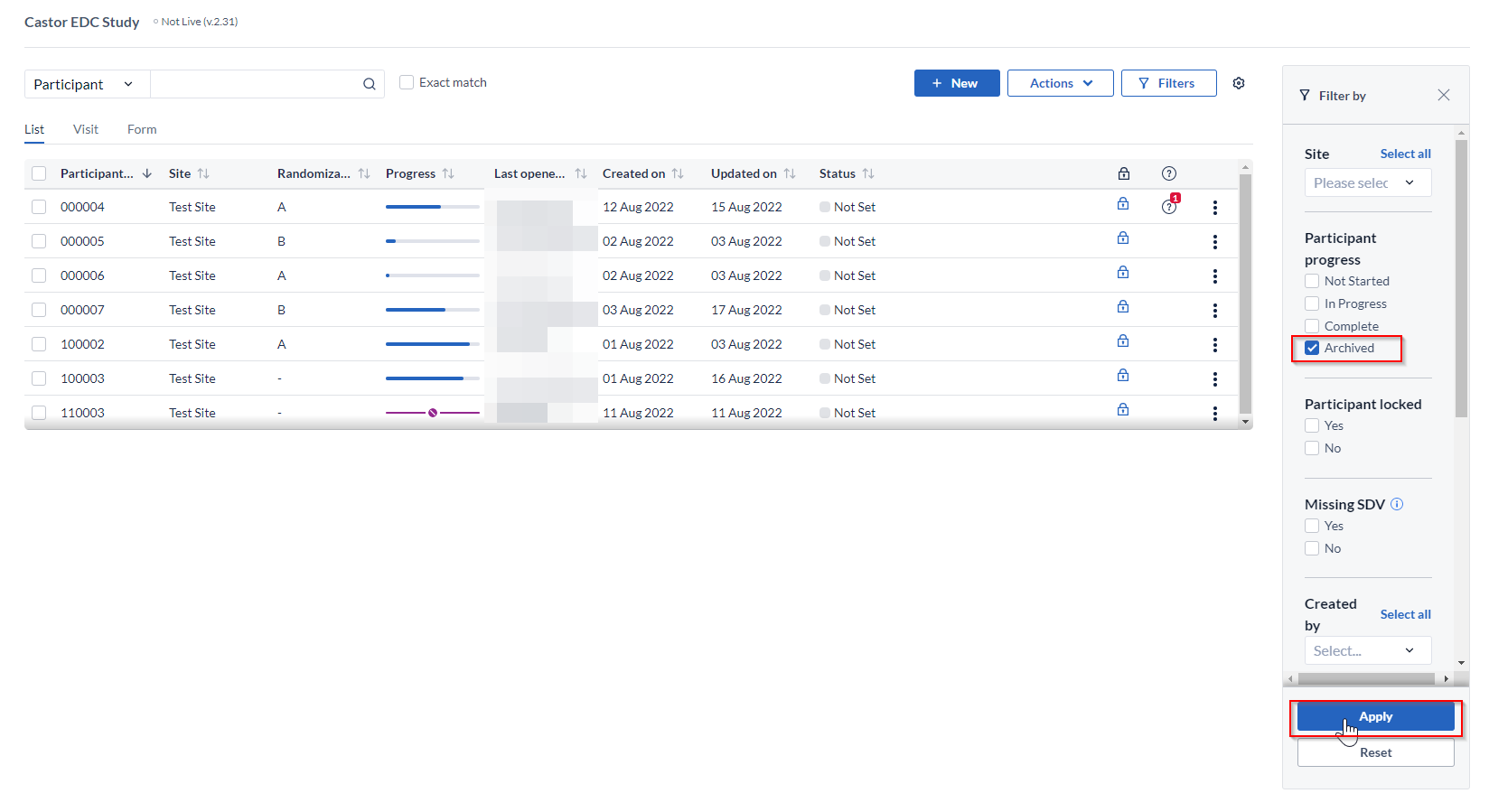
To see archived participants in the Participants tab, click on the 'Filters' button:

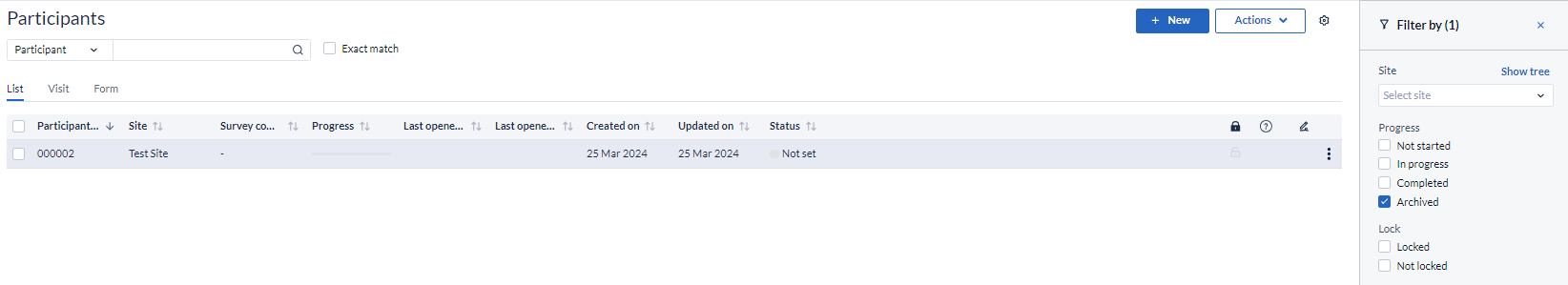
Set Participant progress to 'Archived' in the 'Filter by' panel and click 'Apply' to view the participants:
By default, archiving a participant will not release the ID, thereby preventing its reuse.
An error message will be displayed when trying to create a participant with the same ID:
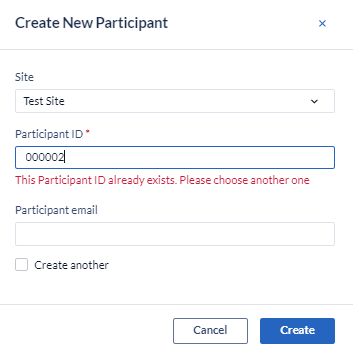
However, there is the possibility of reusing a participant ID, but you will need to request the activation by reaching out to support@castoredc.com.
When this setting is enabled within the study, archiving a participant assigns the prefix 'ARCHIVED-' to the participant ID, so if you create a participant immediately after archiving the previous one, the same participant ID will be used. If you archive older participants, the participant IDs will not be reused.
Archiving surveys or repeating data
To archive a survey or repeating data, open an individual participant and navigate to the tab 'Surveys' or 'Repeating Data' accordingly. Alternatively, open the global 'Surveys' or 'Repeating Data' tab. Click on the cogwheel next to a survey or repeating data and choose the option 'Archive survey' or 'Archive repeating data'.
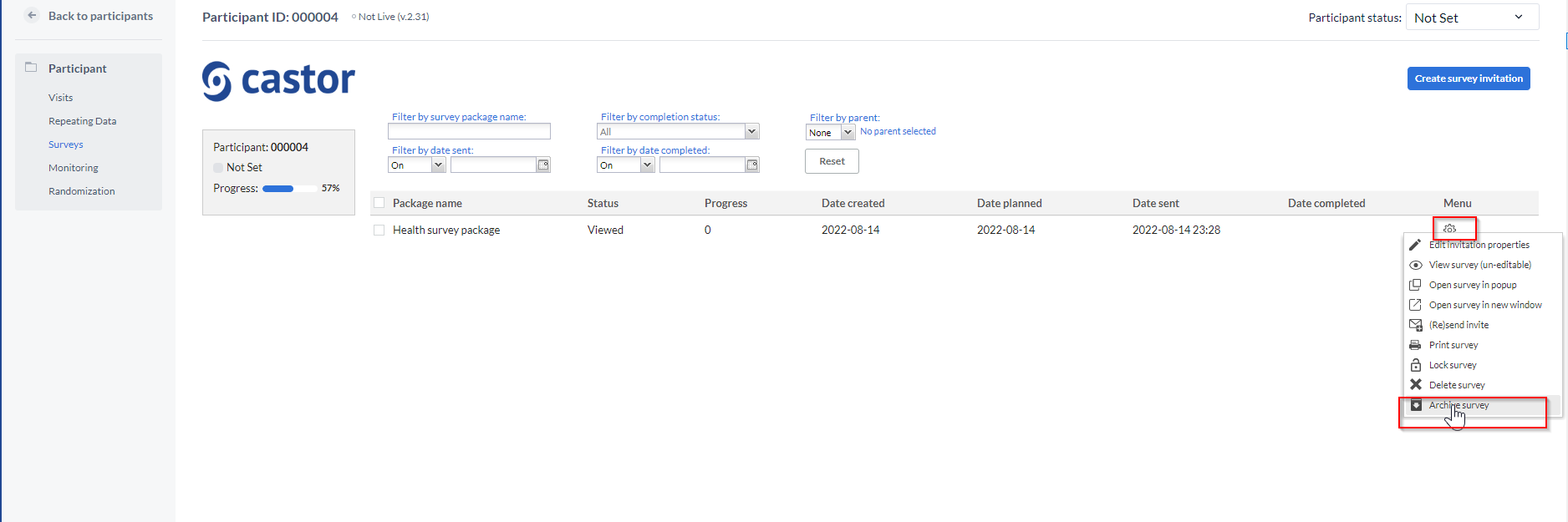
To see archived surveys in the Surveys tab, choose Filter by completion status -> 'Archived'.
To see archived repeating data in the Repeating Data tab, choose Filter by status -> 'Archived'.
Castor EDC/CDMS also allows to un-archive participants, repeating data or surveys. It means that once they are un-archived, they will be moved to an overview of the participants, repeating data or surveys again. To un-archive repeating data or survey, click on the cogwheel next to it and select 'Unarchive survey' or 'Unarchive repeating data'.
Unarchiving a participant
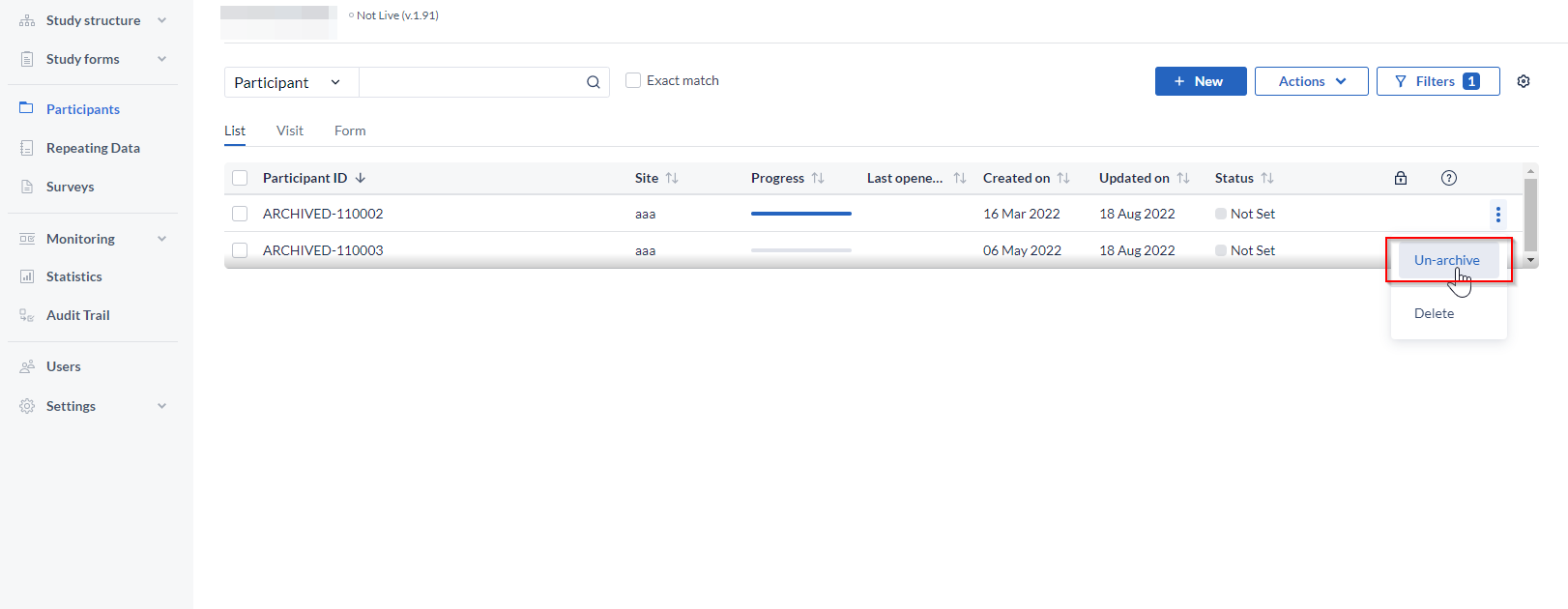
To unarchive a participant, follow the steps below:
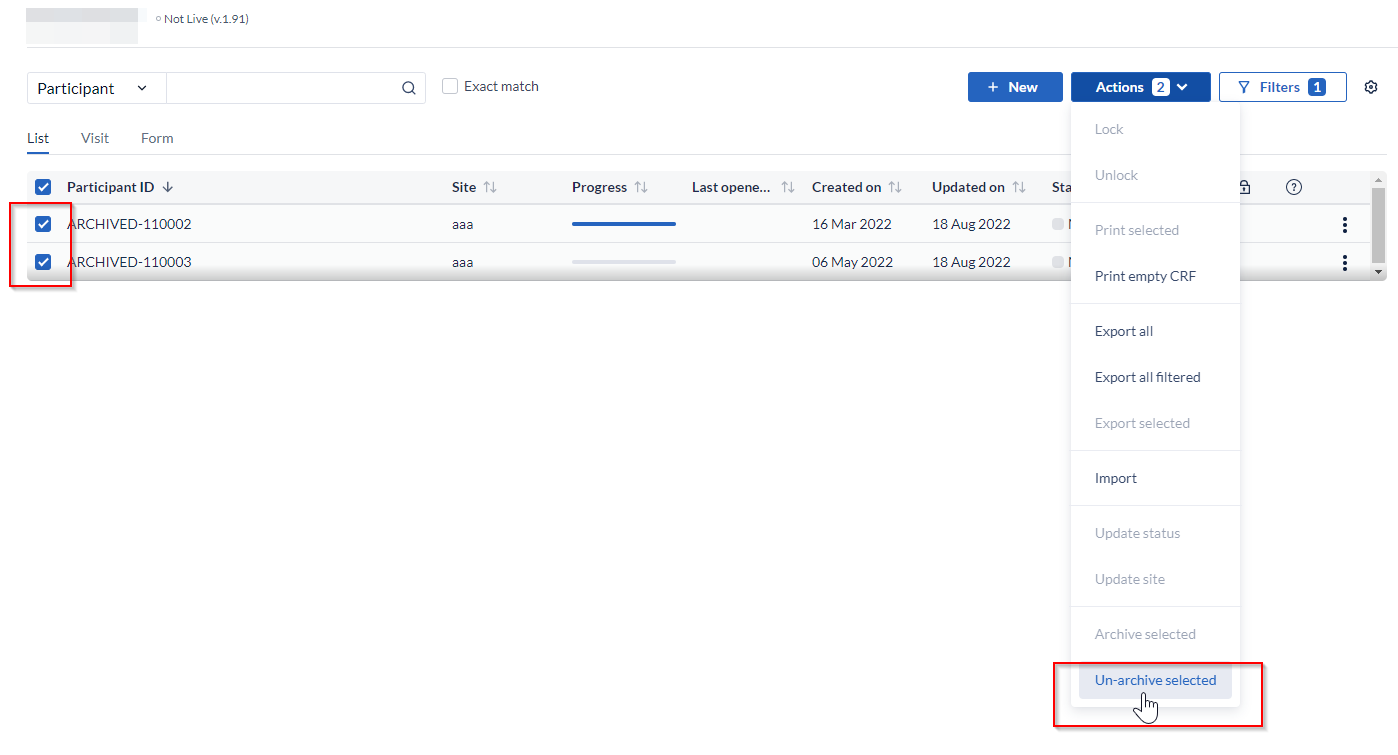
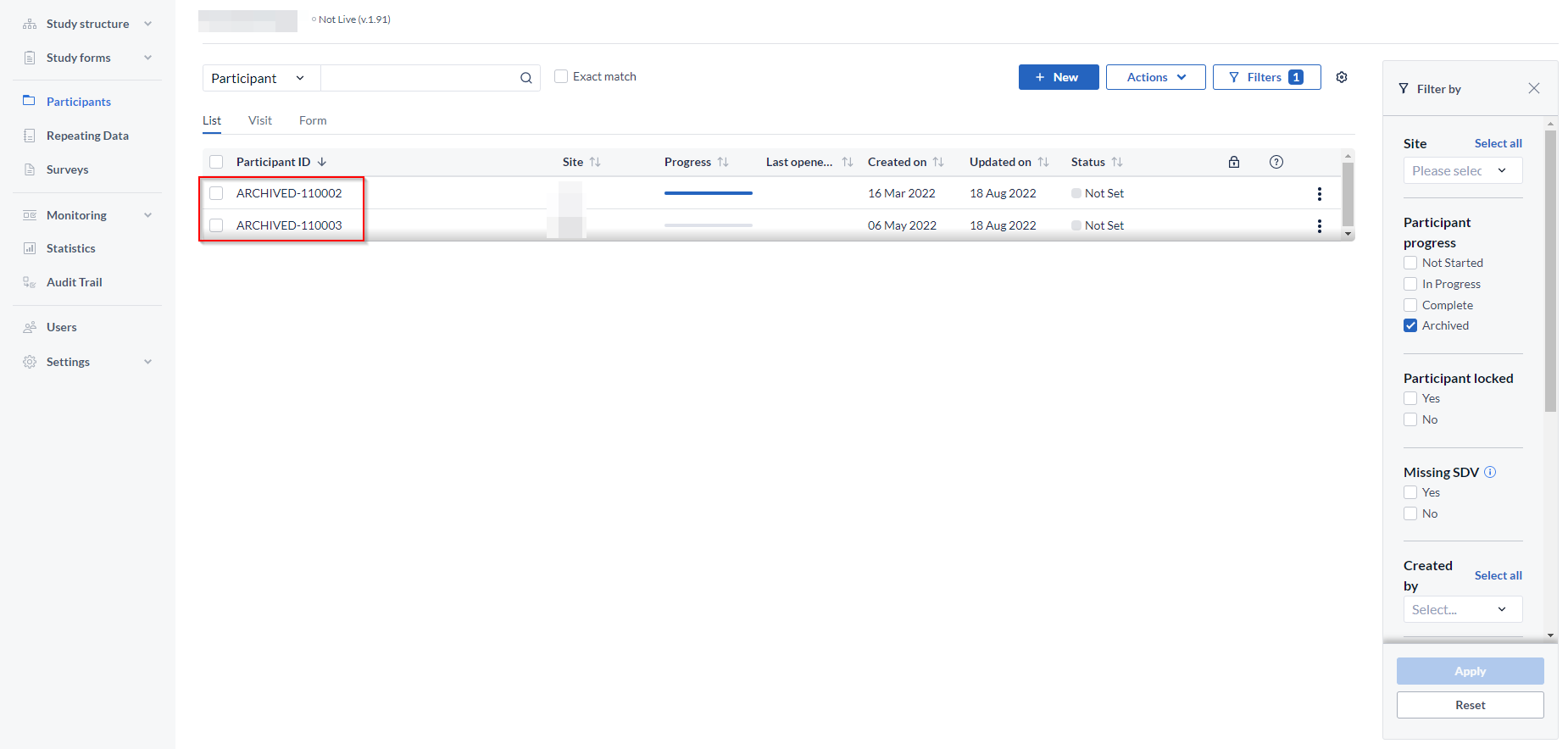
- Click on the 'Filters' icon at the top right - select 'Archived', then 'Apply' to apply changes. An overview of archived participants will be shown.
- To un-archive one participant: Click the 3 dot menu to the right of the participant you would like to un-archive - select 'Un-archive'. The participant will be unarchived and will be visible in the list of all active participants. If the participant ID of the archived already exists in the Participants list, upon un-archiving suffix corresponding to an incremental number will be added depending on the number of participants with the same ID. For example, if there is an archived participant ARCHIVED-110003 and there is an active participant 110003, upon unarchiving the archived participants will appear as110003-1 in the list of active Participants.
- To un-archive participants in bulk: select participants you would like to un-archive, click on the 'Actions' button and choose the option to 'Un-archive selected'.