Delete a participant in CDMS
Table of Contents
Delete a participant
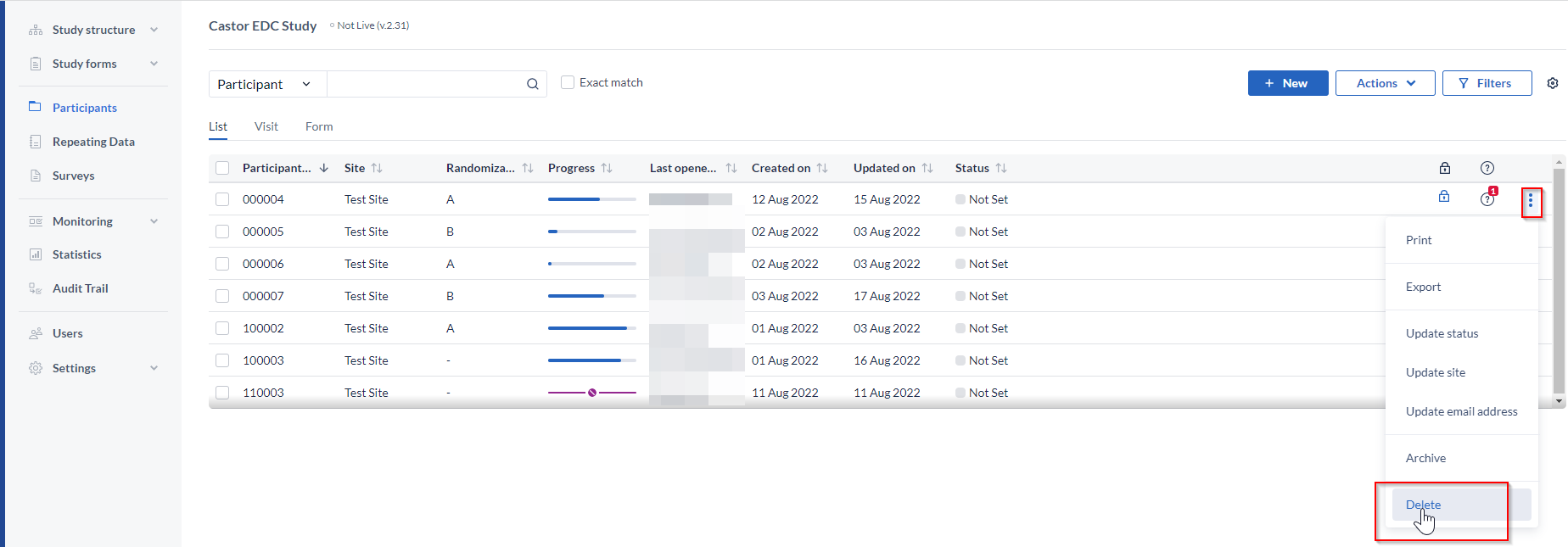
If you are not yet collecting data and your study has never been set to live, you can delete the participant. To delete a participant, click on the 'Participants' tab, then click on the three dots next to the participant you want to delete. Then click 'Delete':
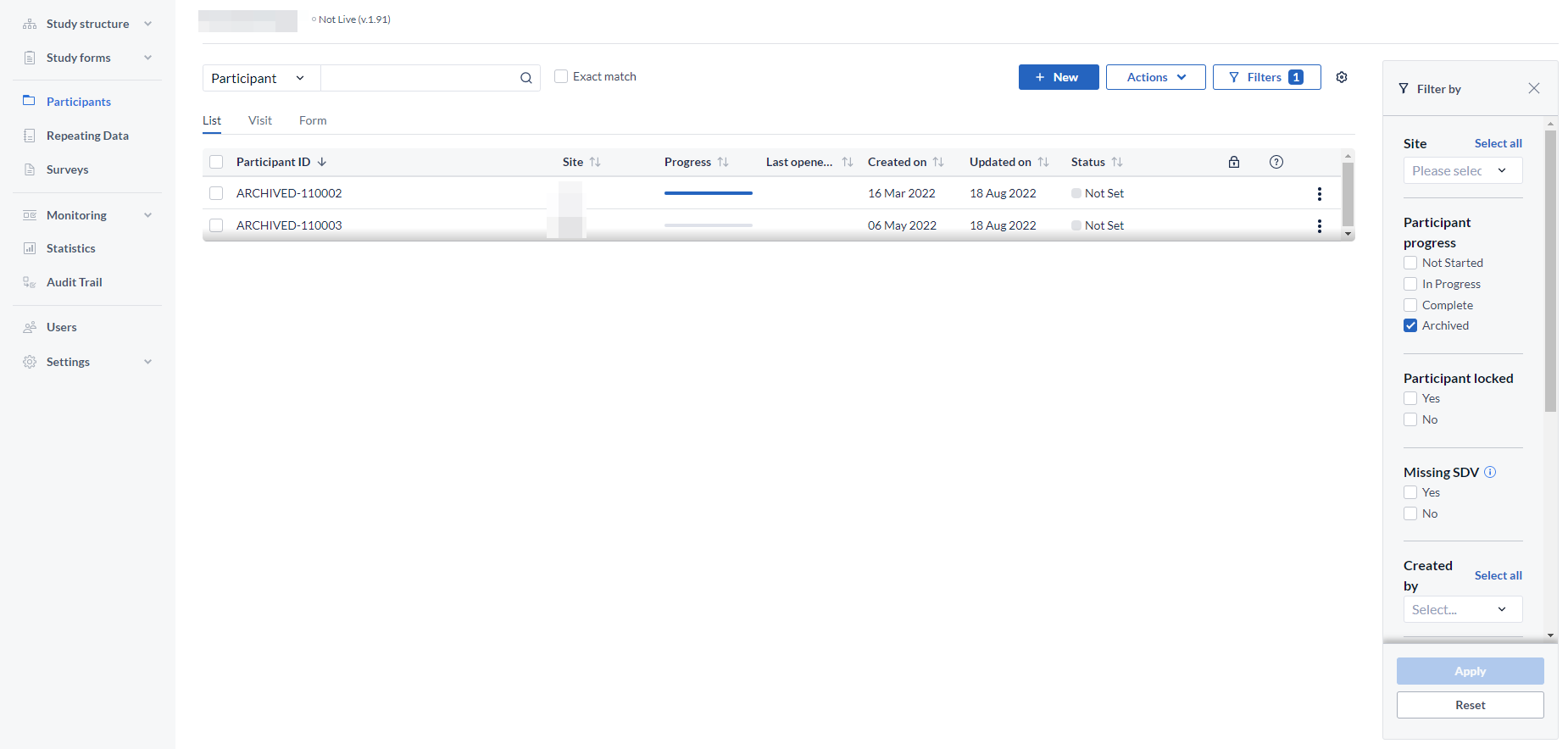
All the participant IDs that are deleted will get the prefix DELETED- and will be logged in the audit trail as such.
For example, if we deleted participant ''110001'' the participant will appear as DELETED-110001 in the audit trail.
Note: We recommend keeping at least one test participant in your study so that you can still test during the study when necessary.
Archive a participant
If you are already collecting data i.e. your study is currently live or has been live once before - it is no longer possible to delete participants and you will need to archive any unnecessary participants. This is due to GCP regulations so that all collected data is traceable. Archived participants will not be exported nor influence your study in any way, but will still be traceable/recoverable in case of an audit.
You can archive a single participant using the three dots menu in the participant overview. To archive multiple participants, select the participants you wish to archive, click on the 'Actions' button and choose the option 'Archive selected':
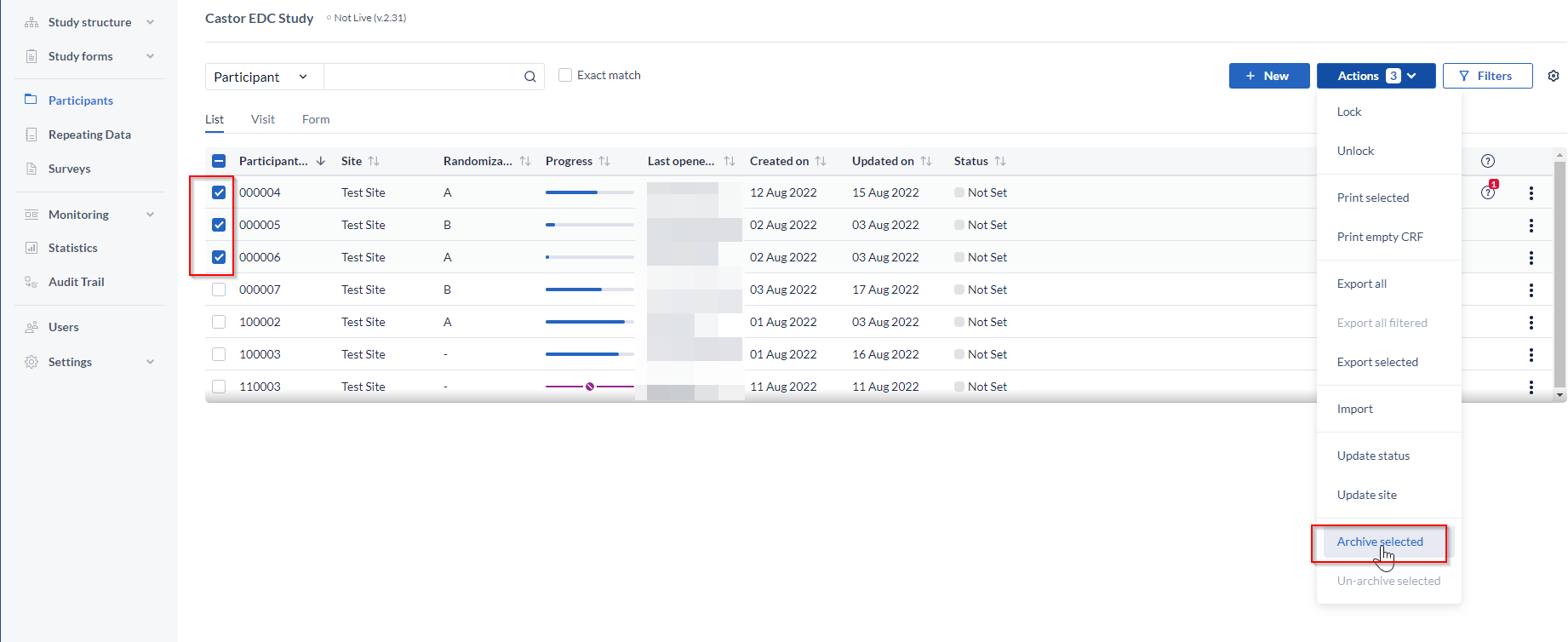
Enter the reason for archiving the participants.
To un-archive archived participants, choose the option 'Un-archive selected' participants in the 'Actions' menu.
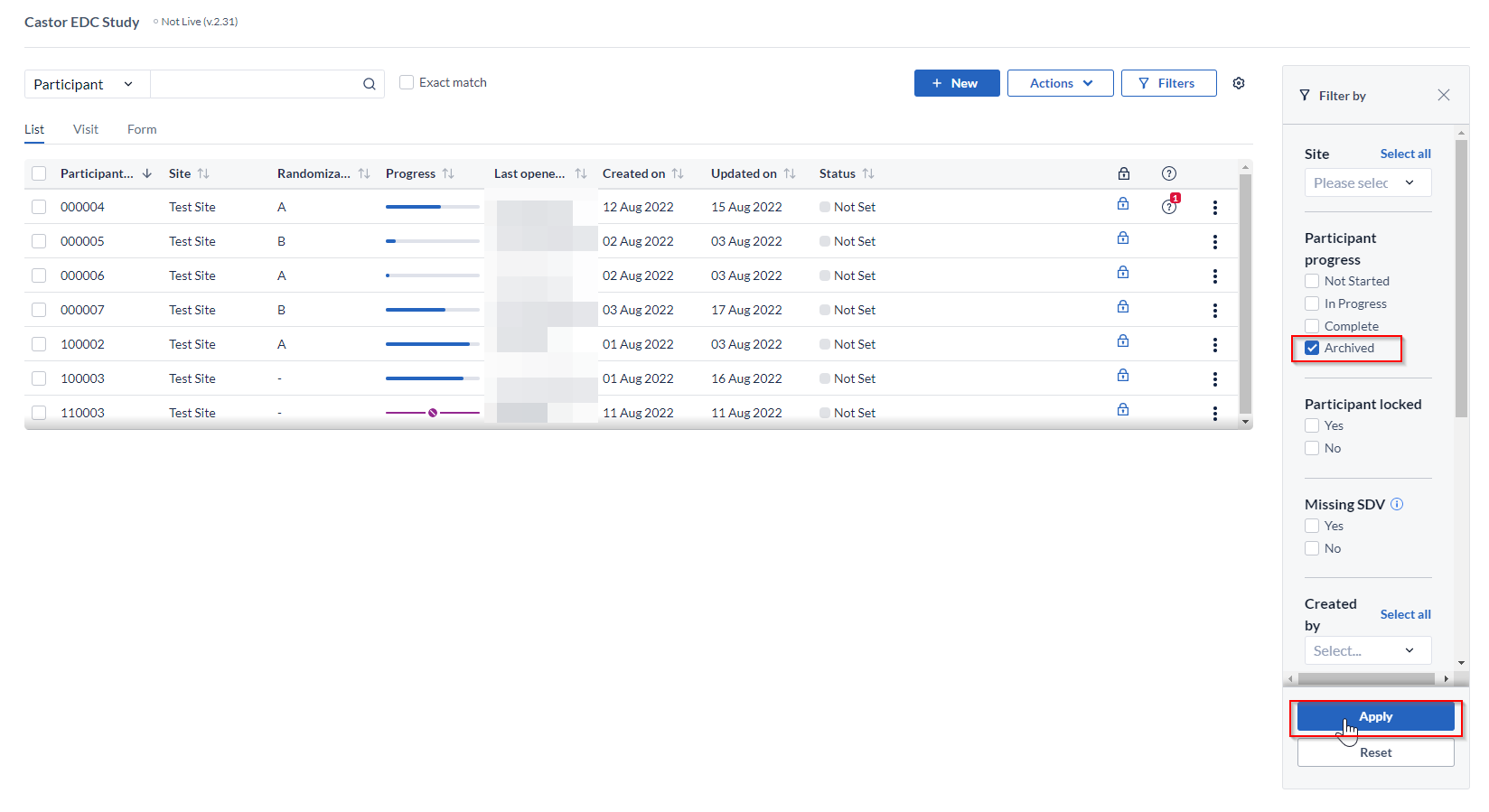
To view archived participants, click on the 'Filters' button:

Select the 'Archived' box in the 'Filters' menu and click 'Apply' button:
Archived participants obtain the prefix ARCHIVED-. The original participant ID will no longer be used.