Creating multiple participants at once in CDMS
Table of Contents
It is also possible to create multiple participants ID at once by importing them.
First, you need to prepare your data horizontally, with the desired participant Id(s) and the site abbreviation (as set in Settings) in one of the first five columns (A-E).
Importing data can only occur into participants and fields that exist in your study and have variable names assigned. The variable names in your import file must be the same as defined in your study. Your import file cannot be empty, at least one valid data point (value) is required. Make sure to include a variable and data value for participant IDs you wish to import.
In the Import dialog select the columns containing the participant Id and the column with the site abbreviation.
The first row should always contain all the variable names.
Save your data as a .csv file.

Once your file is ready, click on 'Import' under ‘Actions’ to import data for multiple participants on the participant's tab.
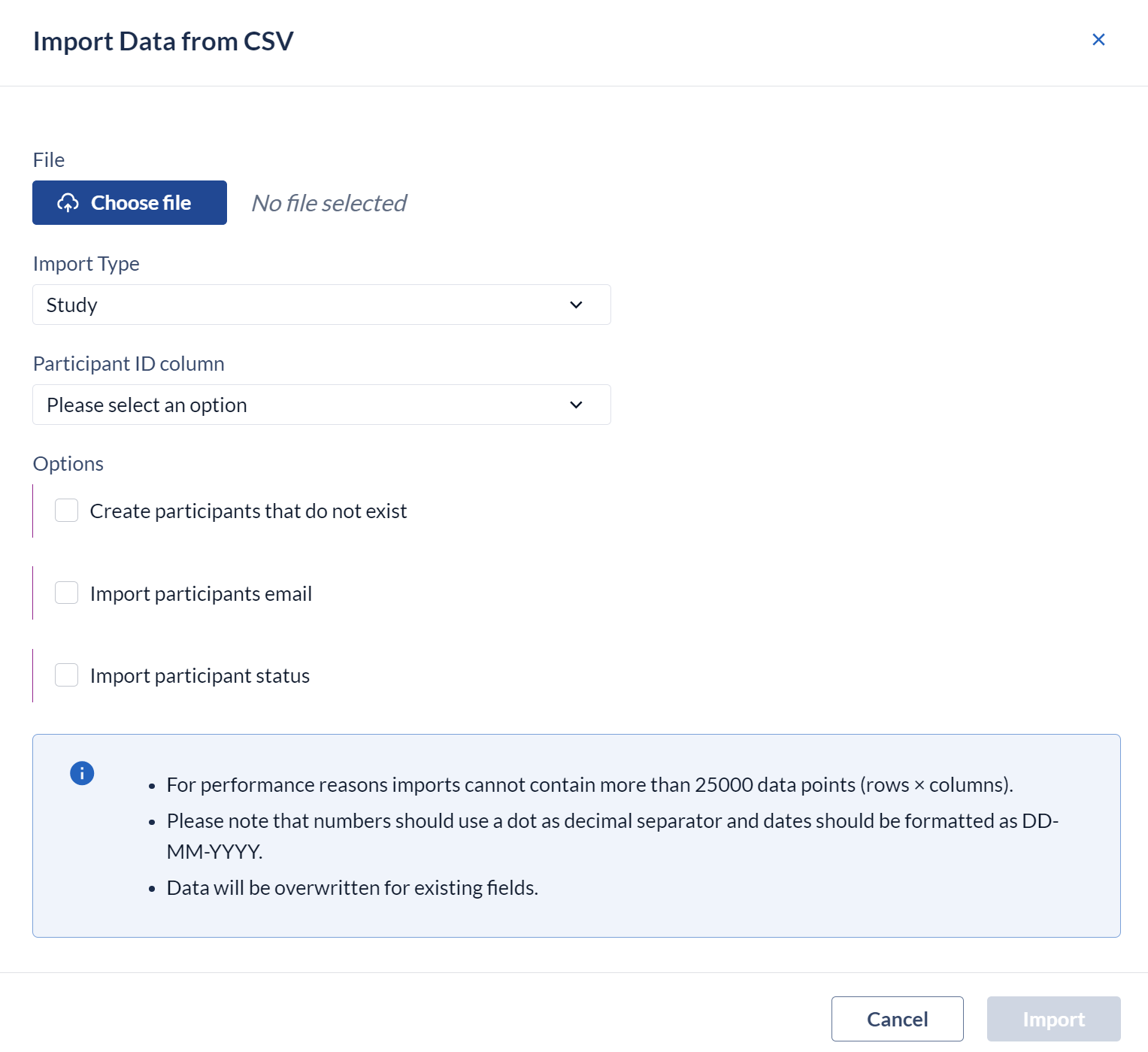
Choose the file and orientation
- File: Choose the file (newer browsers will always display c:\fakepath as a security measure).
- Import type: Choose study import type
- Participant id column: Choose the column from your csv-file that contains the participant ID's.
- Create participants that do not exist: Check the box to create new participants with the imported data
- Site abbreviation column: Choose the column from your csv-file that contains the abbreviation of the site
- Import email addresses: Choose if you want to import email addresses
- Click Upload
The data importer will show a preview of all data that will be imported, this way it is possible to see the results before it is actually imported and allows for cancelling the import if needed. clicking Accept button will continue the data import.