Exporting full study structure and data before applying changes in CDMS
Before merging the changes to the production environment, it is possible to download a full backup of the production study. The full backup includes a copy of the study structure and the study data for all participant records.
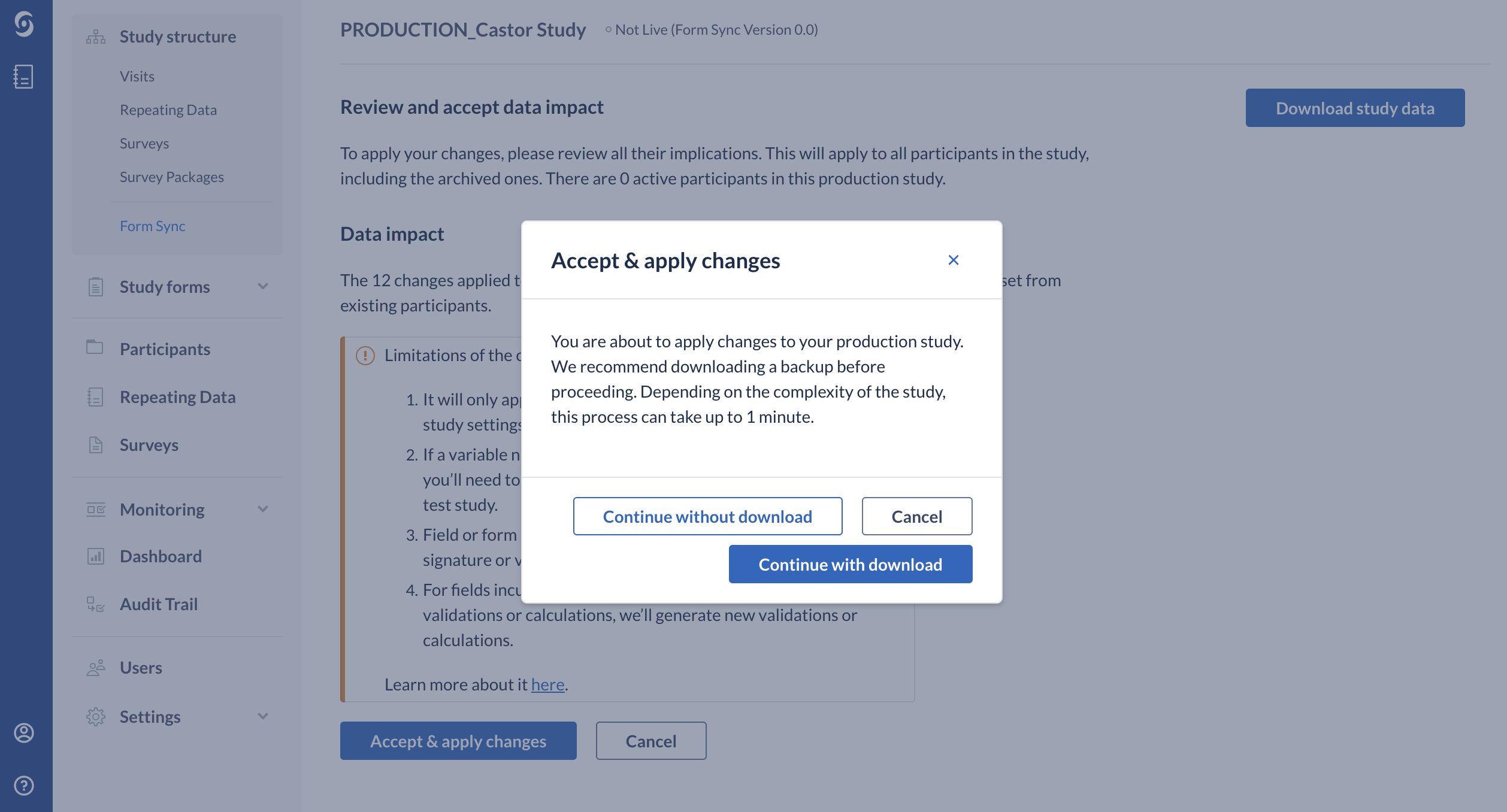
The option ‘Download backup’ is only available for users with full Institute rights, including view randomization, decrypt and view survey data.
This means that the study team member who will be performing the downloading of the backup needs to have all rights enabled across all institutes and not just export rights across all institutes. For example, if the encryption module is enabled, the study team member needs to have Decrypt rights enabled.
If all rights are not granted to the study team member performing this operation, the export file will not be complete (eg. hidden fields due to missing View Randomization rights).