Merging changes from test environment to production environment in CDMS
Table of Contents
Learn how to safely and efficiently merge changes from a test environment to a production environment in CDMS in the article below.
Final changes are always applied from the test to production study, never the other way around. Once the changes are applied to the production environment, it is no longer possible to revert them. To remove any changes that have been applied you will need to open the test study, reverting the changes in the test study manually and merging these changes to the production environment.
Reviewing changes and data impact in the production study
Once the changes are finalized in the test study, navigate to the production study. Follow the steps below to merge changes from the test study to the production study:
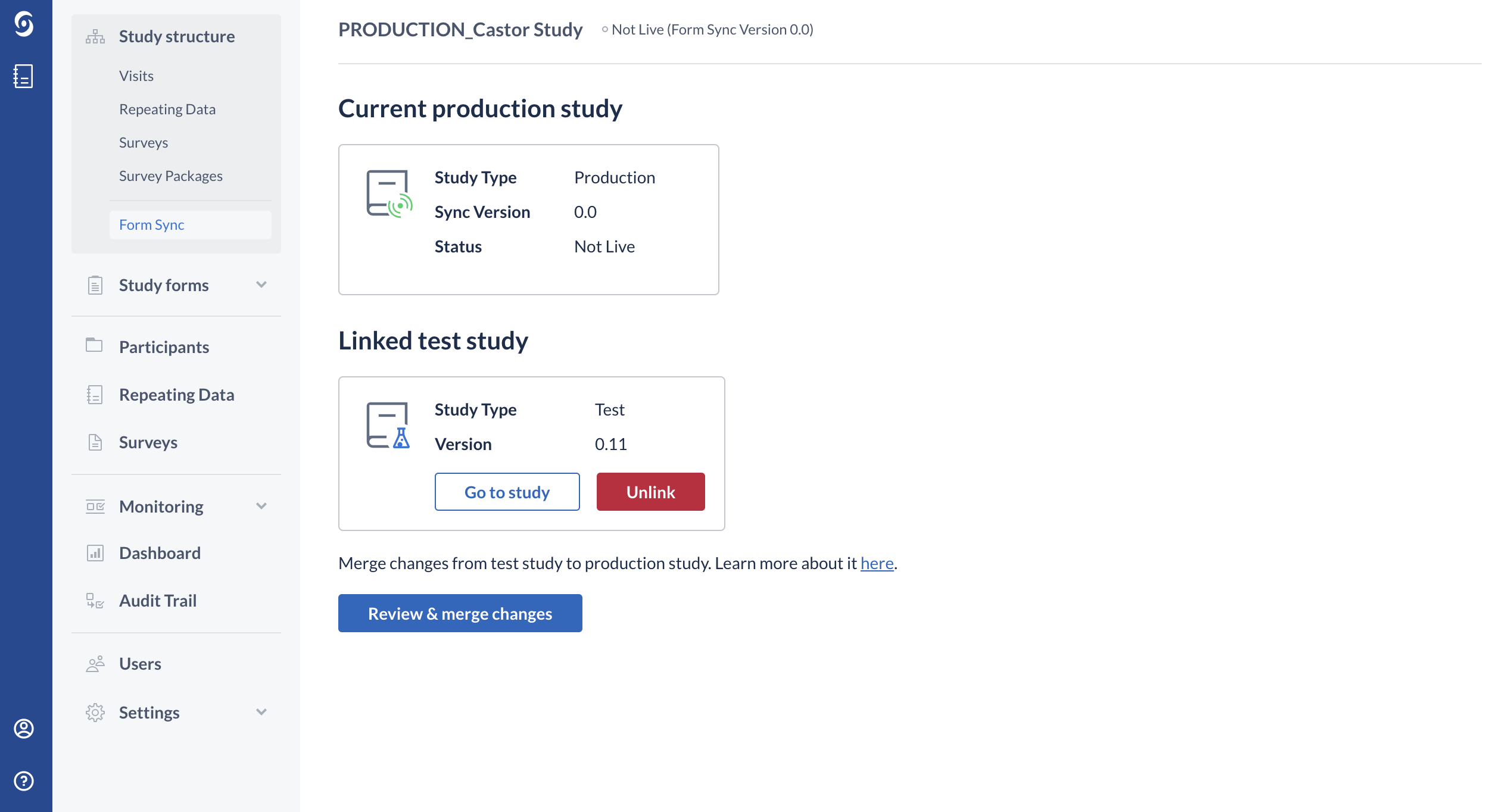
1. Navigate to the 'Study Structure' tab -> 'Form Sync' sub-tab.
2. Click on the 'Review Merge Changes' button:
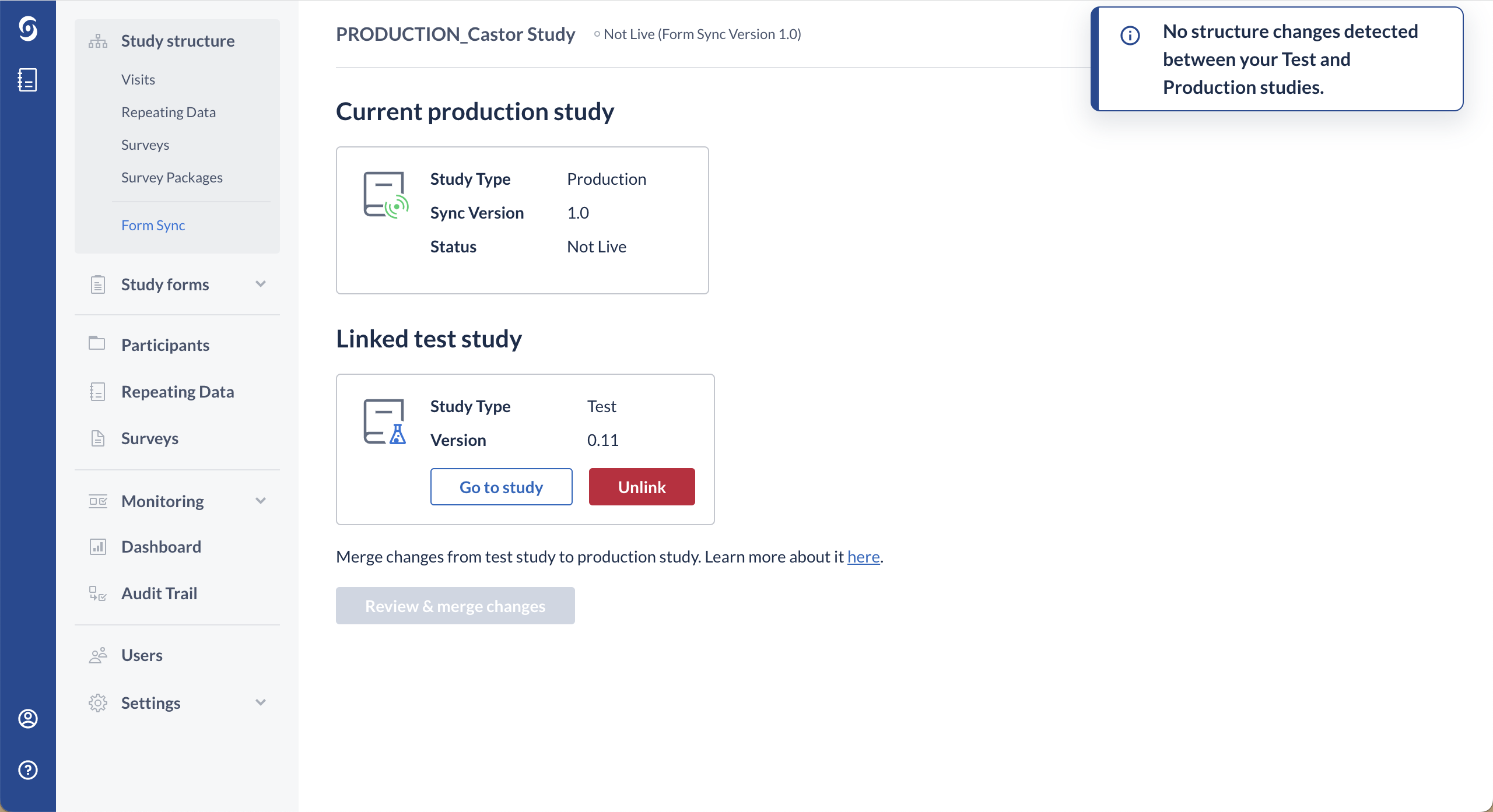
3. If there are no changes detected, a message will appear informing you that no changes have been detected in the test study:
4. If changes to the test study are detected, 'Review Changes to Production' page will appear listing all the changes in the test study. The default 'Table' view, which shows the summary of detected changes:
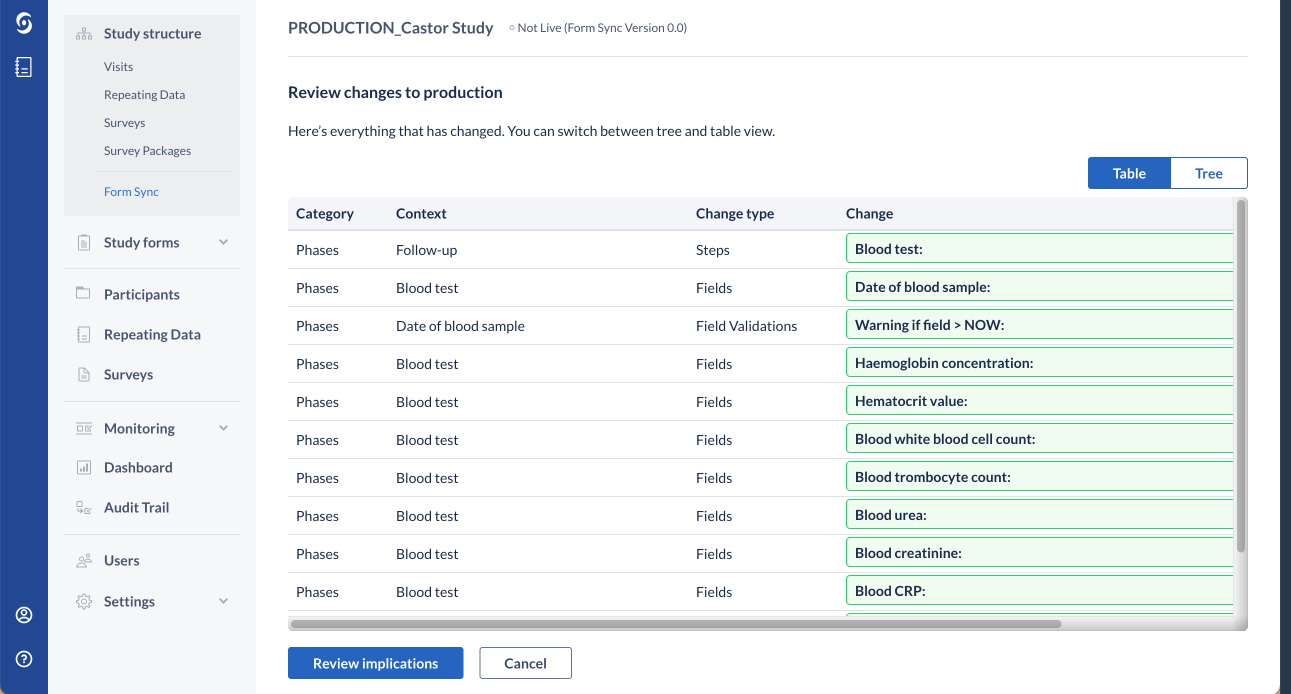
The 'Tree' view displays the entire study structure of the test study including the detected changes. Use buttons 'Collapse All', 'Expand Updated' or 'Expand All' to either expand or collapse the study structure and check which changes have been detected.
It is possible to switch to the 'Table' view, which shows the summary of detected changes:
5. Press the ‘Review implications’ button. The 'Review and accept data impact' page displays participants that currently exist in the production environment, and data impact with indication of the number of data points and number of participants that are affected by each change.
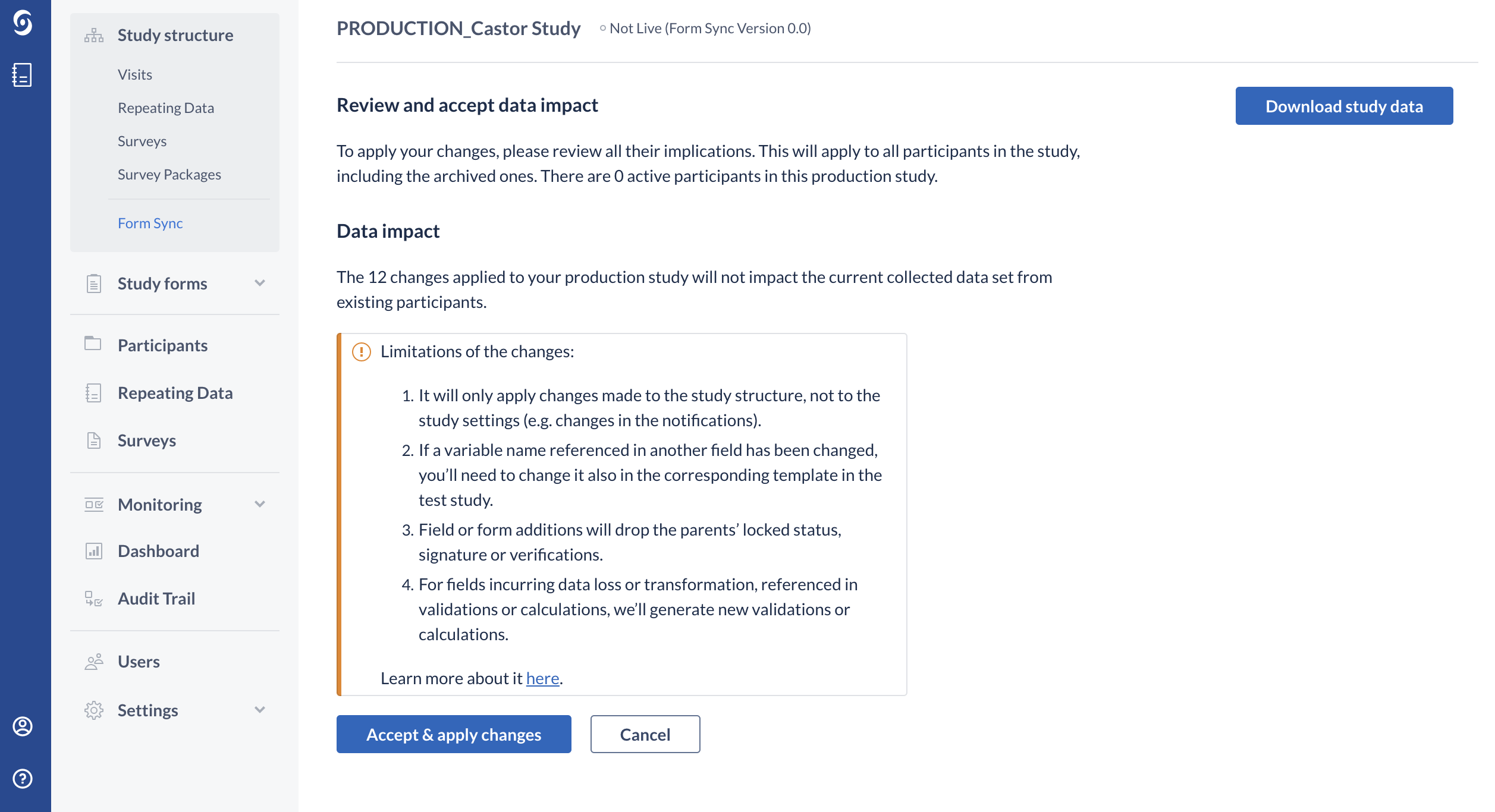
6. To confirm and finalize your structure updates, you will need to review and accept all of the data changes. The changes will apply to all existing participants in the study, including locked and archived participant records:
7. Click on the ‘Accept & apply changes’ button to apply changes. A dialog window 'Accept and Apply Changes' will appear.
8. Choose to either 'Continue with download' or 'Continue without download' or 'Cancel'.
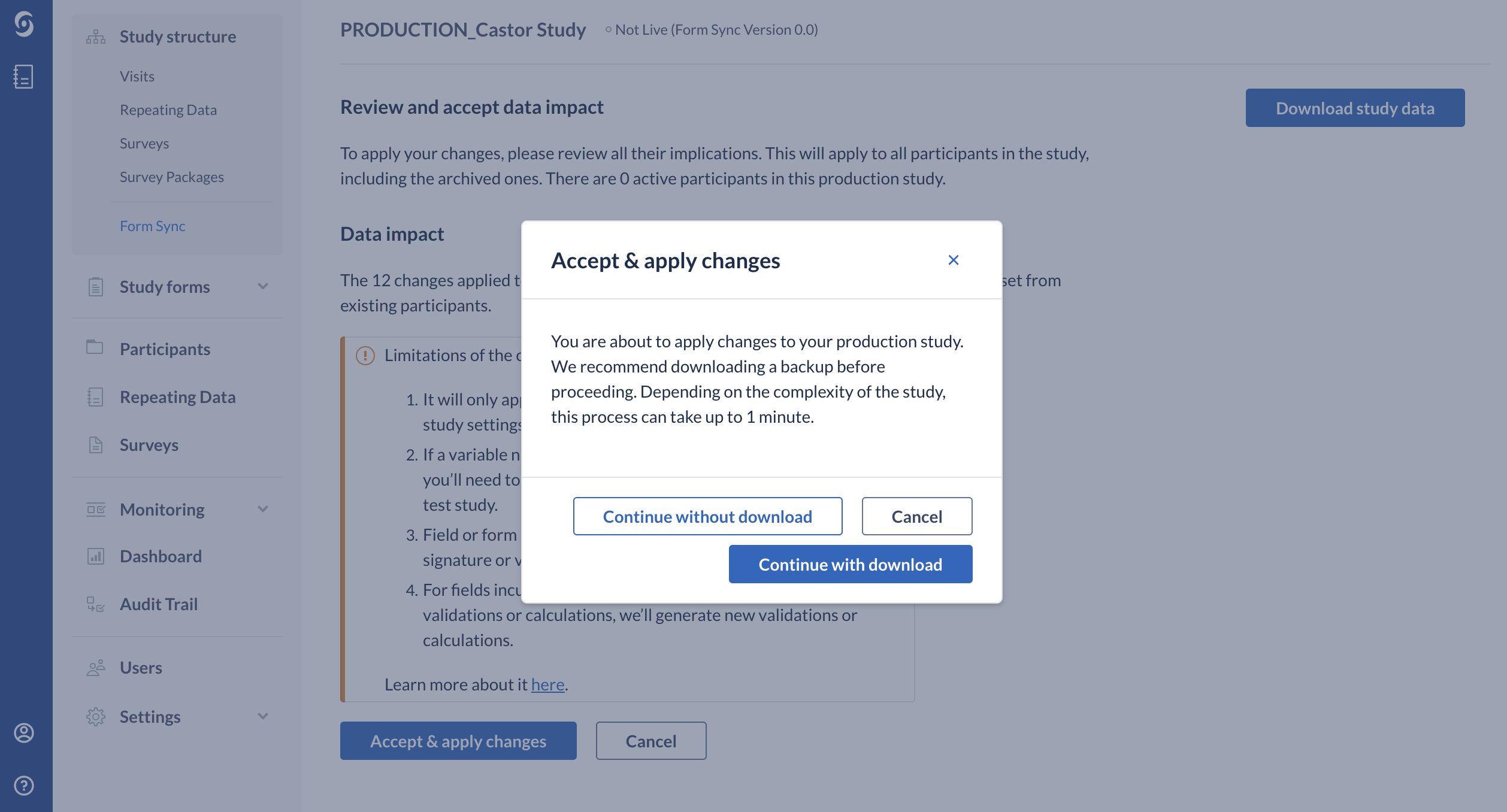
- Choosing the option to 'Continue with download' will download the full backup (including data) of your study. Please note that the study team member who will be performing the downloading of backup needs to have all rights enabled across all institutes and not just export rights across all institutes. For example, if the encryption module is enabled, the study team member needs to have Decrypt rights enabled. Read more information about the download here.
- 'Continue without download' option means that the changes will be applied to the production study without downloading the full backup of your study. 'Cancel' option will allow you to cancel the process of merging changes from your test study to the production study.
Versioning of the study structure
Form Sync version number is a production study form version and represents the number of previously applied changes from the test to the production environment. The version number increases every time new changes have been merged successfully.
Allowed changes
-
Only changes made to the test study structure will be applied to your production study.
This excludes changes to the study settings such as changes in the automation engine and notifications. -
Changes to fields, visits, and forms will automatically drop the SDV, Signature, Custom verifications, and Lock statuses.
- This includes: updating field labels, updating field variable names, reordering fields in forms, updating field properties
-
This excludes: updating visit labels, reordering forms in visits, adding a new option in an Option Group, updating the configured field validation(s)
Based on the Castor CDMS 2024.4.x.x a new report will be available for studies that have a SDV Plan active: Interim Data Impact Report for Mid-Study Updates
Users can now generate an interim data impact report during the Form Sync process, providing detailed insights into structural changes, data loss, and SDV (source data verification) impacts. Additional enhancements have been made to the ‘Data Impact’ page to improve visibility into cumulative data loss across all study instances.

A ‘Generate Interim Report’ button is now available on the initial Form Sync page for production studies and it will contain 4 files, in CSV format:
- structural-changes.csv: Overview of all detected structural changes.
- collected-data-impact-summary.csv: Summary of the impact on collected data, highlighting potential data loss.
- collected-data-impact-per-participant.csv: Participant-specific view of the impact on collected data.
- sdv-impact-per-participant.csv: Participant-specific view of the impact on SDV at the field level.
- For fields incurring data loss or transformation, where the field values are referenced in validations or calculations, new validation messages or calculated values will be generated. Each participant record and form which contains validation messages and calculated values will need to be opened to update these values.
- For variable name changes where the variable name is referenced in summary and calculation fields, the new variable name will need to be reflected in the summary or calculation template in the test study.
- It is not possible to change the field type using form sync. This is to only allow compatible field types and avoid/limit data loss.
- The option groups which are not in use in the Production study will be deleted when merging the changes.