Metadata Annotations Settings in CDMS
Table of Contents
Castor CDMS supports the storage of metadata with your fields, so you can enrich your data set with metadata like SNOMED codes, LOINC codes, or whichever type of metadata you want. To learn more about metadata, follow this link.
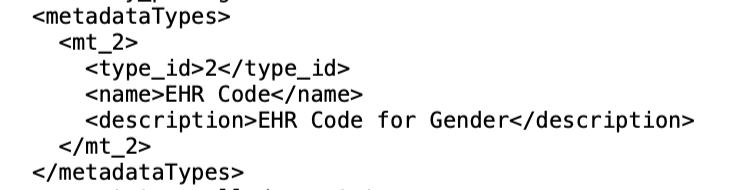
Define Metadata Annotation Types
In the Settings tab, click on the sub-tab ‘Annotations’ where you can manage all your study's metadata annotations settings:
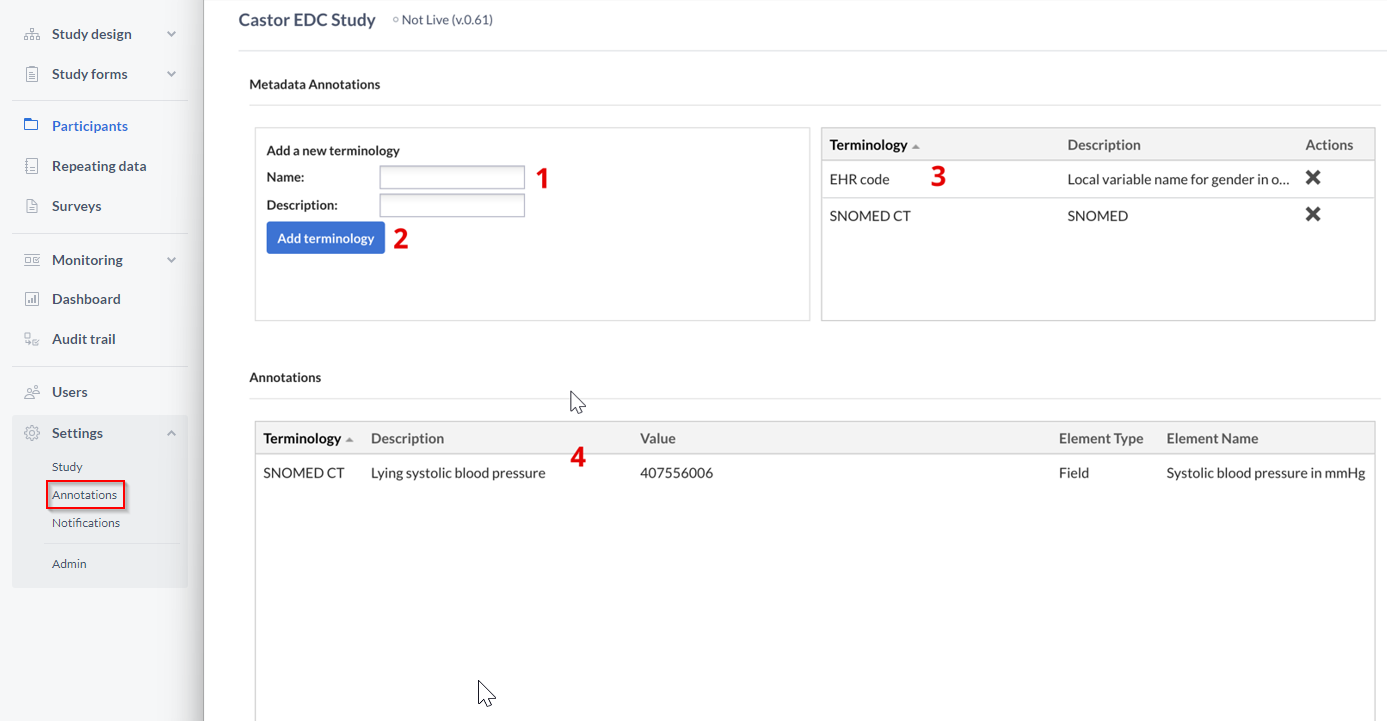
To add a new terminology (metadata type), for example SNOMED, follow these instructions:
- Enter the name and description.
- Click on the button ‘Add terminology’ to save the changes.
- All defined terminology will appear in the right panel.
- All fields associated with metadata will be shown in the bottom panel of the Settings in the ‘Annotations’ section.
Save metadata for a field
Once you have defined terminology, you can save it for a given field. For example, you can add the necessary terminology for blood pressure in your ‘Systolic blood pressure in mmHg’ field:
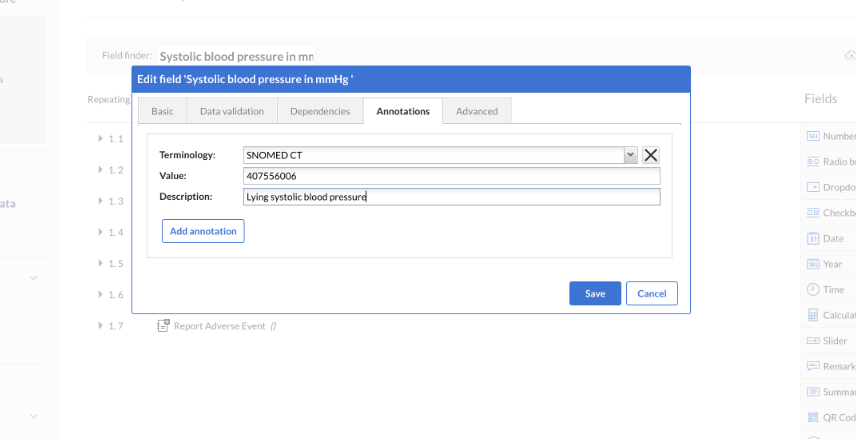
To save metadata for a field you follow these instructions.
Exporting metadata
The metadata annotations can be exported by exporting your study structure for each field:
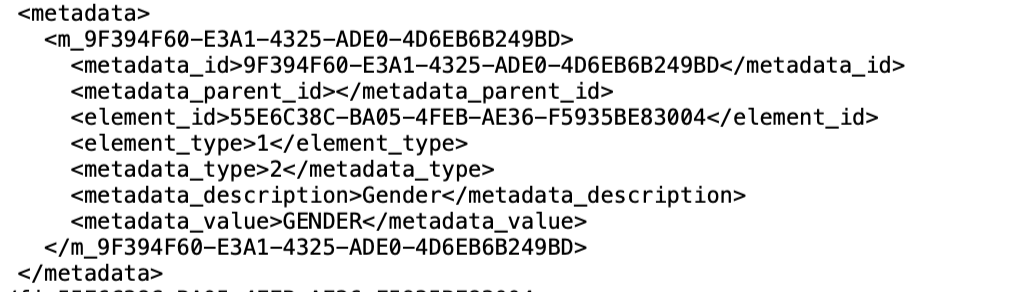
It is also possible to see all the metadata types defined for a particular study: