Notifications for Serious Adverse Events in EDC/CDMS
Table of Contents
Castor's Notification settings allow for the creation of email notifications for specific repeating data types. Often users want to create a notification for serious adverse events or those Adverse Event repeating data structure (AE) where it was indicated that an event may have been related to study participation. The standard notification system only allows a notification on the creation of an AE repeating data instance and not on specific data that was entered in the repeating data instance.
To get around this, we will use the "repeating data structure within a repeating data structure" in order to create a notification for only those AE repeating data instances that are labeled serious.
Create Serious Adverse Event (SAE) repeating data
Create an SAE repeating data with a repeating data type "Other". This is in addition to your normal Adverse Event repeating data.
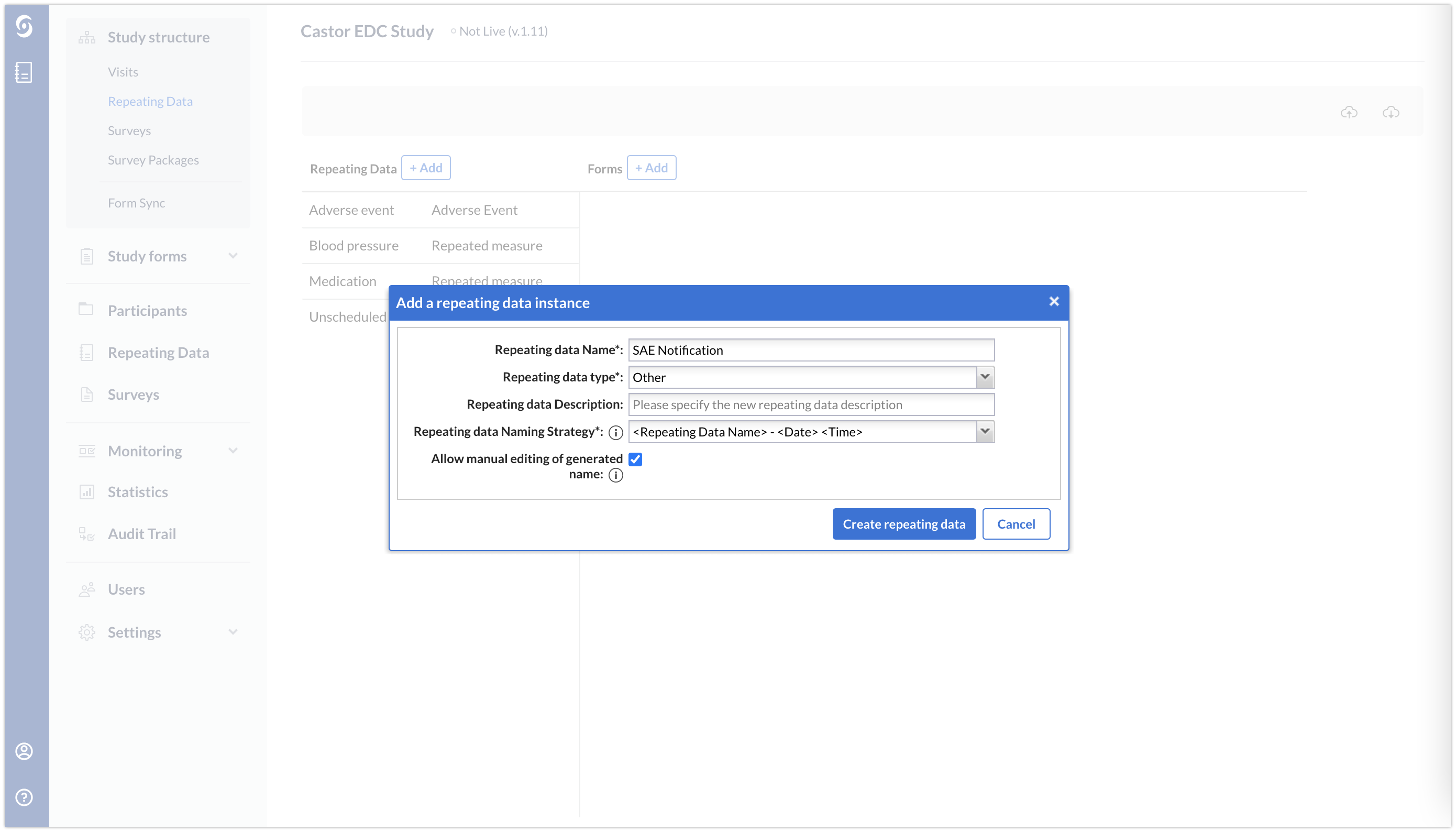
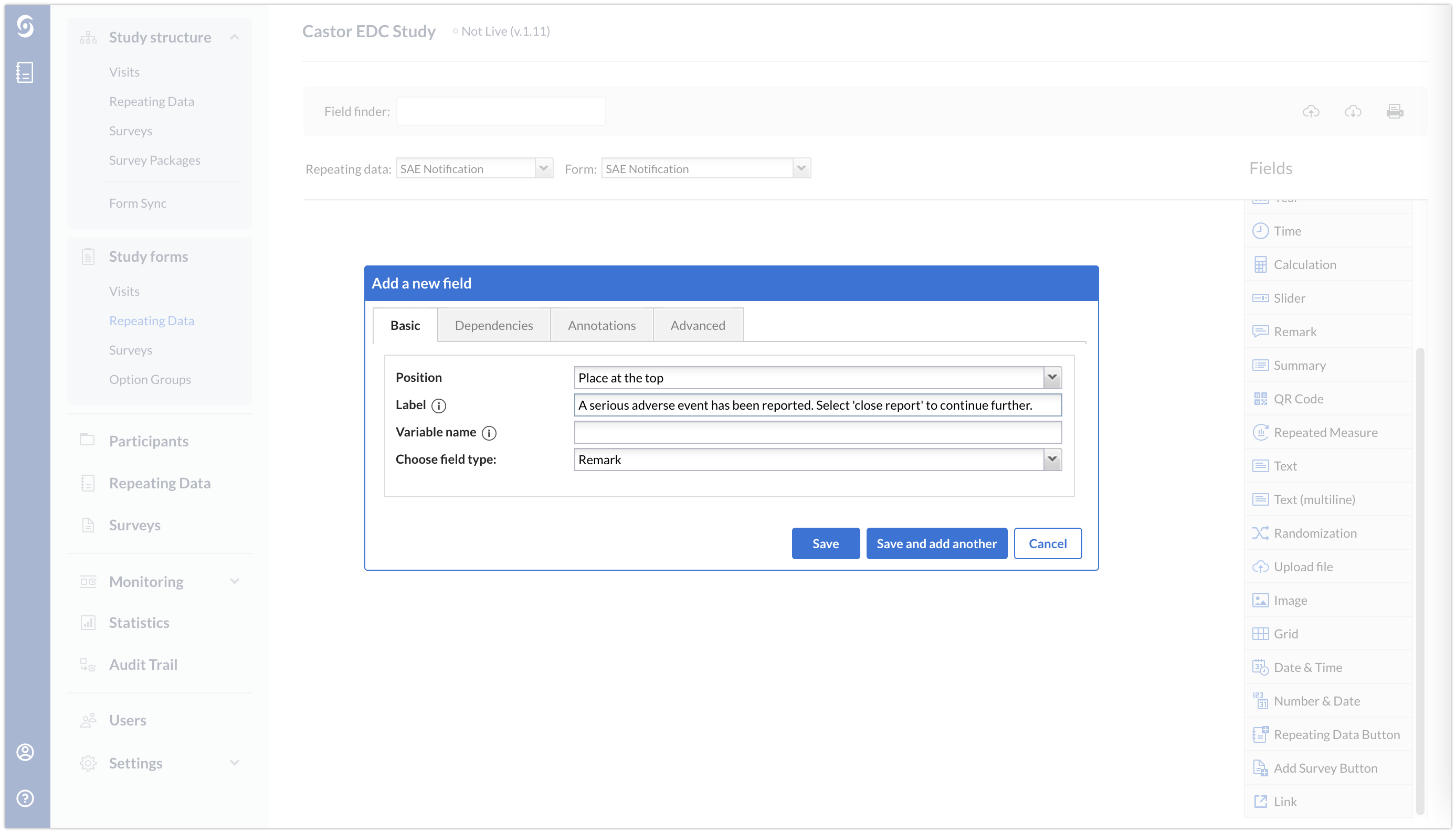
The SAE repeating data will not contain any data. It will only include a remark field providing instruction to the data entry user.
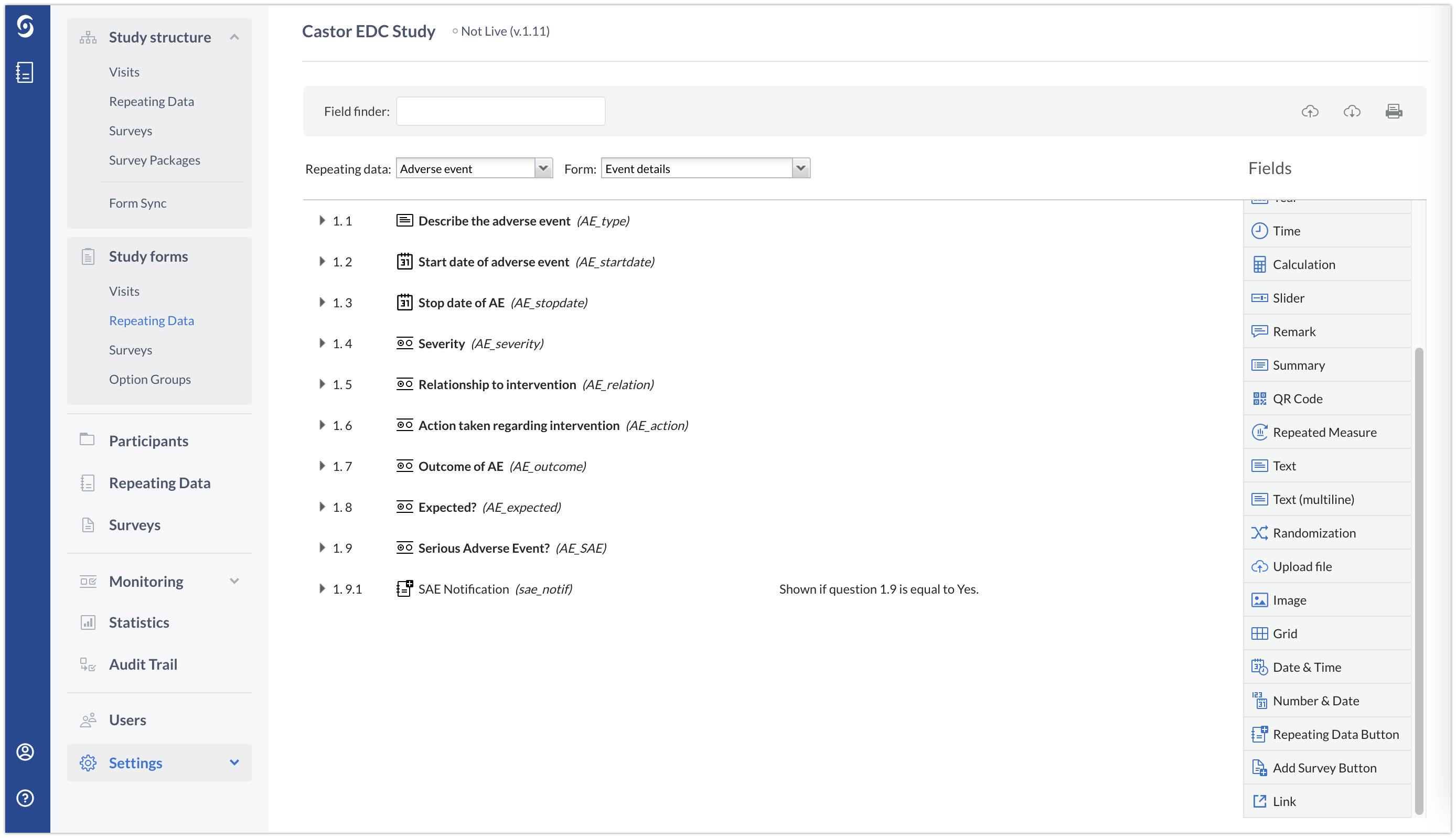
Adverse Event repeating data
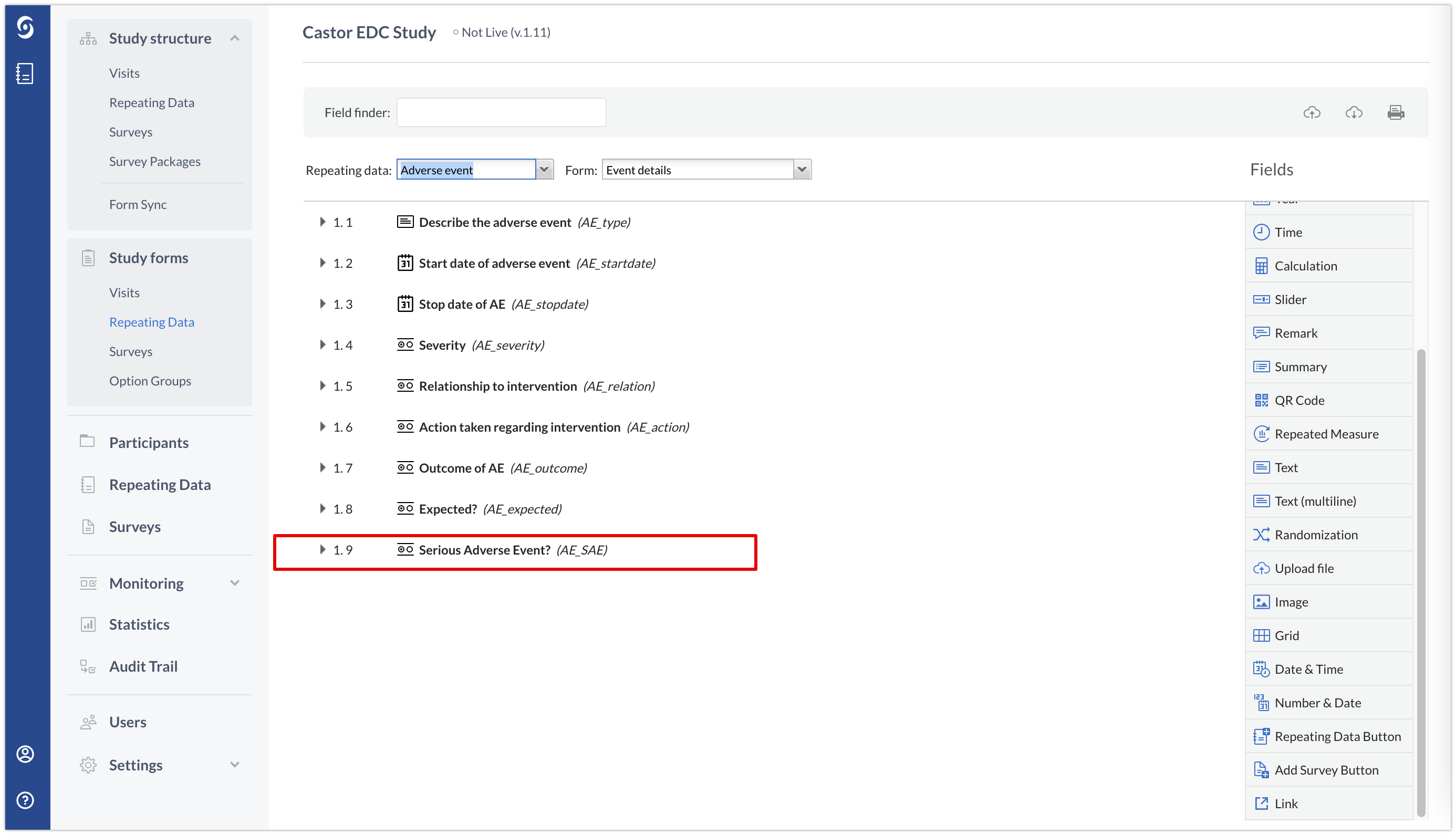
1. Navigate to your Adverse Event repeating data in form builder. You can ddd a calculation using if/else logic that will inform us if the selected criteria was chosen. For, if "Serious" was selected, or study personnel indicates that the Adverse Event may be related to study participation, then a value of "1" will be returned. You may choose to hide this calculation once you have confirmed that it is working properly.
Alternatively, as in the example below, you can create a radiobutton field where a data entry study team member can add information whether it is an SAE or not.
2. Next, insert a repeating data button and link the button to the SAE repeating data that you previously created. This button should be dependent on your calculation field being equal to 1 or on the radiobutton field.
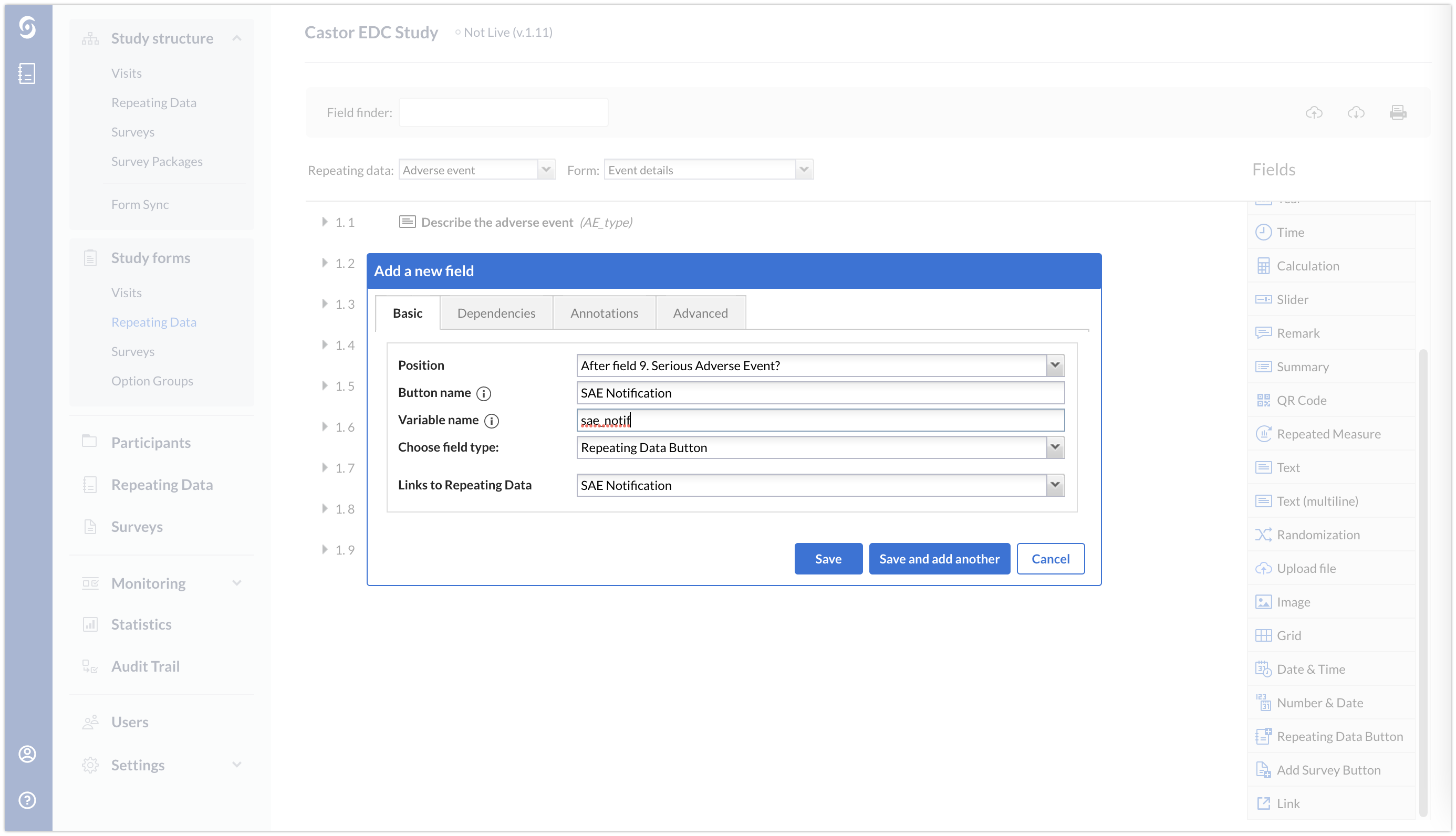
The final form will look as follows:
Create Notification
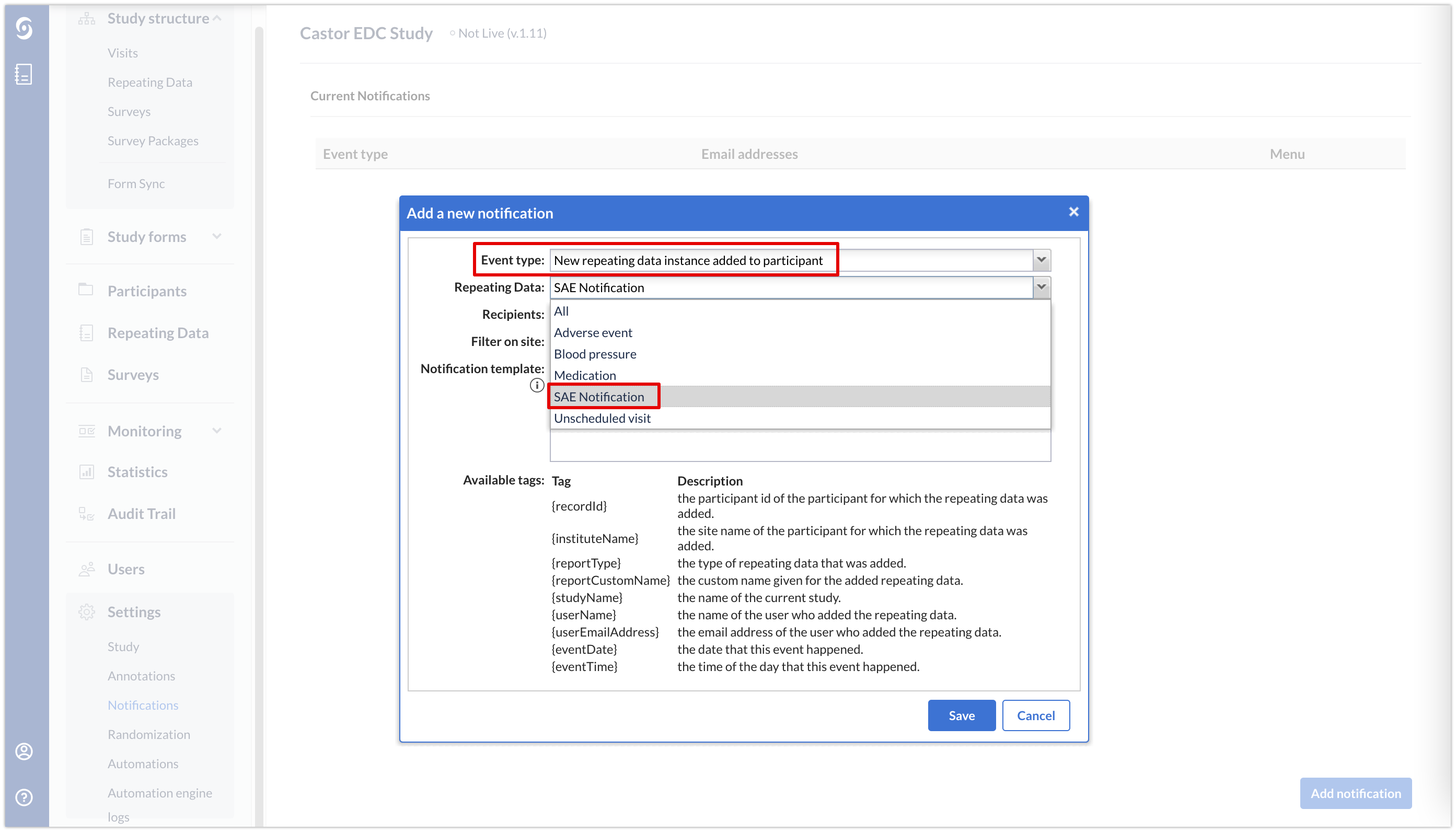
In your study settings, you can create a repeating data notification on the SAE repeating data.
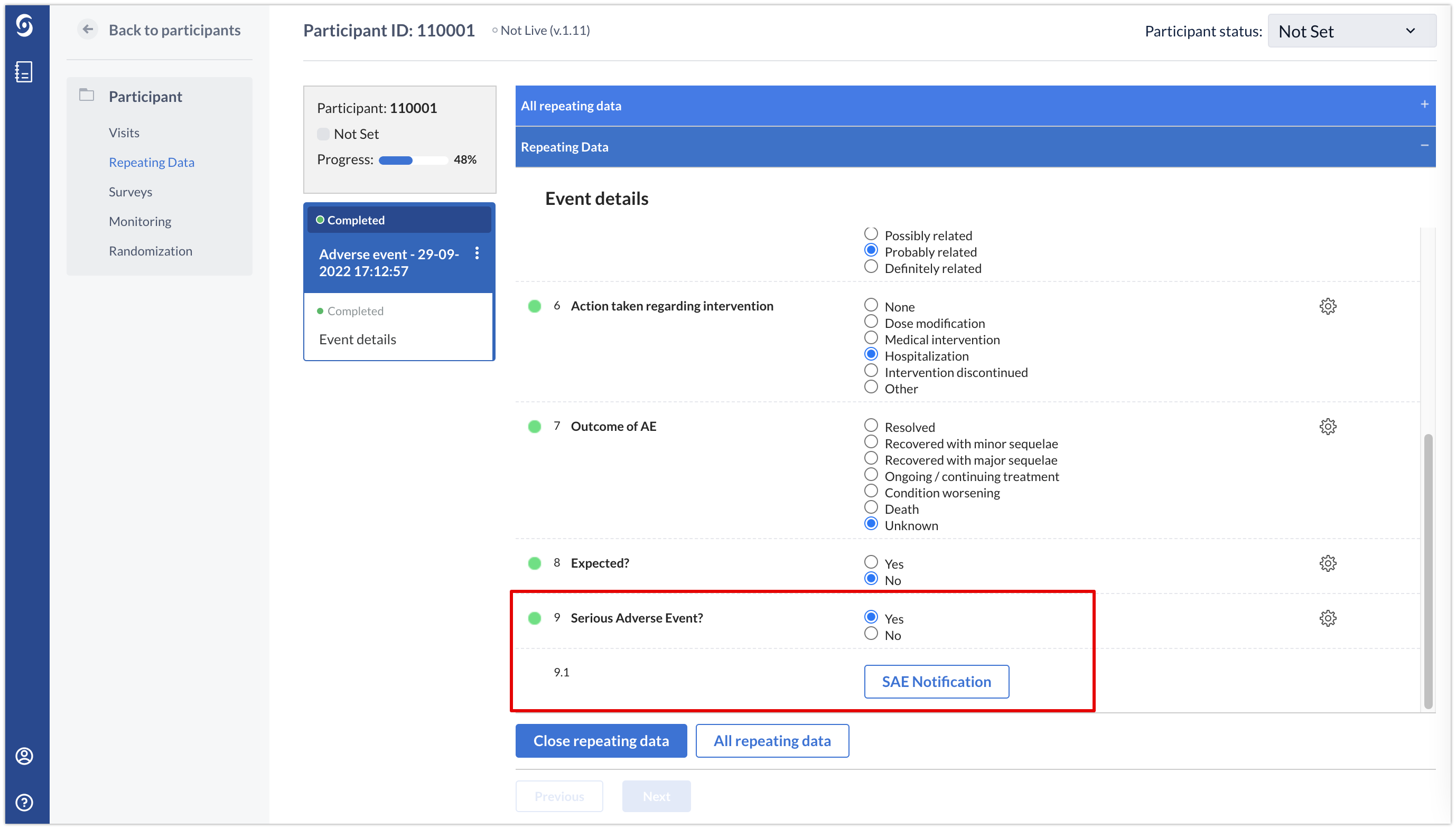
In the data entry view, once the field ‘Serious Adverse Event?’ is set to ‘Yes’ a button will appear to create a notification.
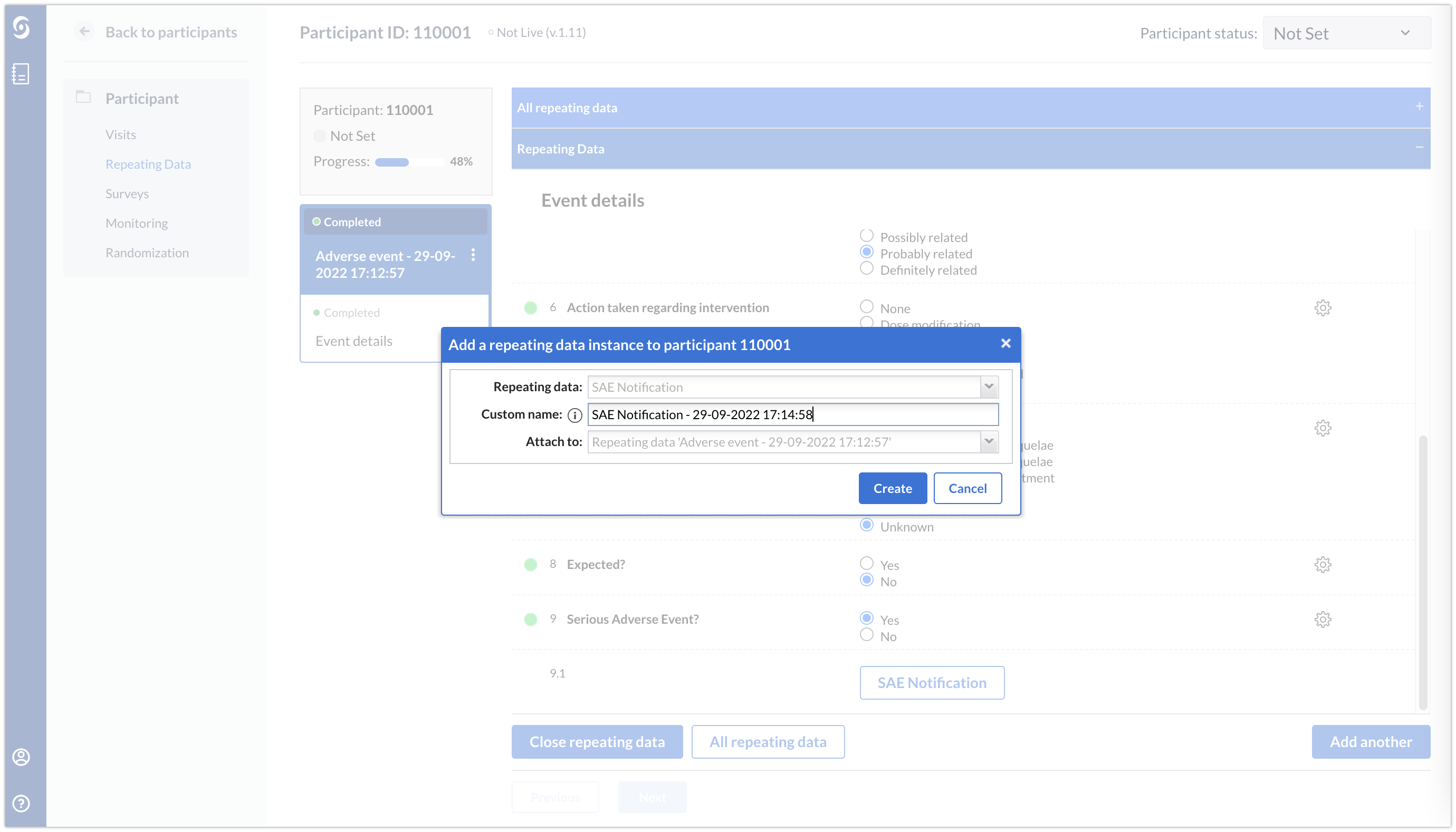
A data entry team member will need to click on the button and create an SAE notification report to trigger an email notification.
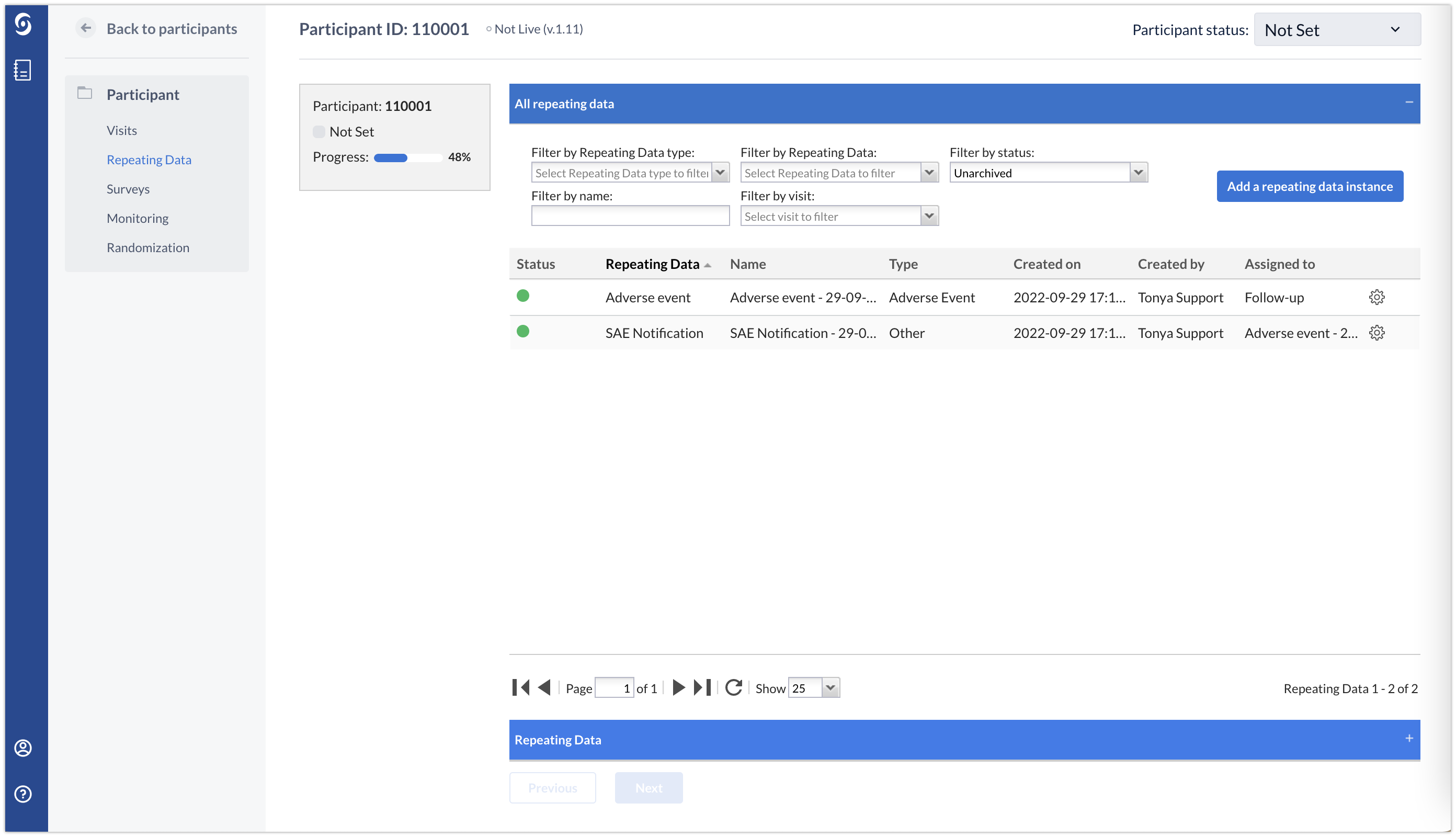
The Repeating data tab
The participant repeating data (shown on the screenshot below) overview and the global repeating data tab will contain both the Adverse Event repeating data and the SAE notification repeating data structure. Note that you can filter this list by selecting Adverse Event or SAE repeating data from the 'Filter by repeating data' drop down.
Please note that this method should only be used to trigger a notification for Serious Adverse Events. You should continue to collect your data in the initial Adverse Event repeating data to avoid having two repeating data structures for the same event.