Using the participants portal in eConsent
Table of Contents
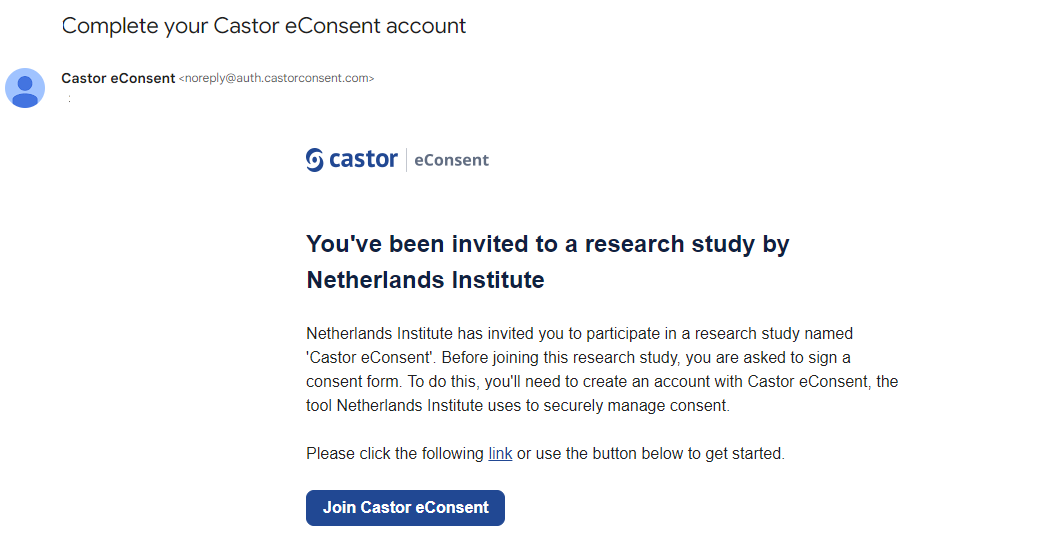
Participants can register their interest in the study through a customized enrollment page, after which they receive an email prompting them to sign up in the Castor eConsent portal. Alternatively, identified patients can be invited directly by the study team (through email) to sign up for eConsent.
Participant registration and eConsent access
When a participant has been added to the eConsent study, an email invitation will be sent to the participant to allow them to register and access the eConsent form.
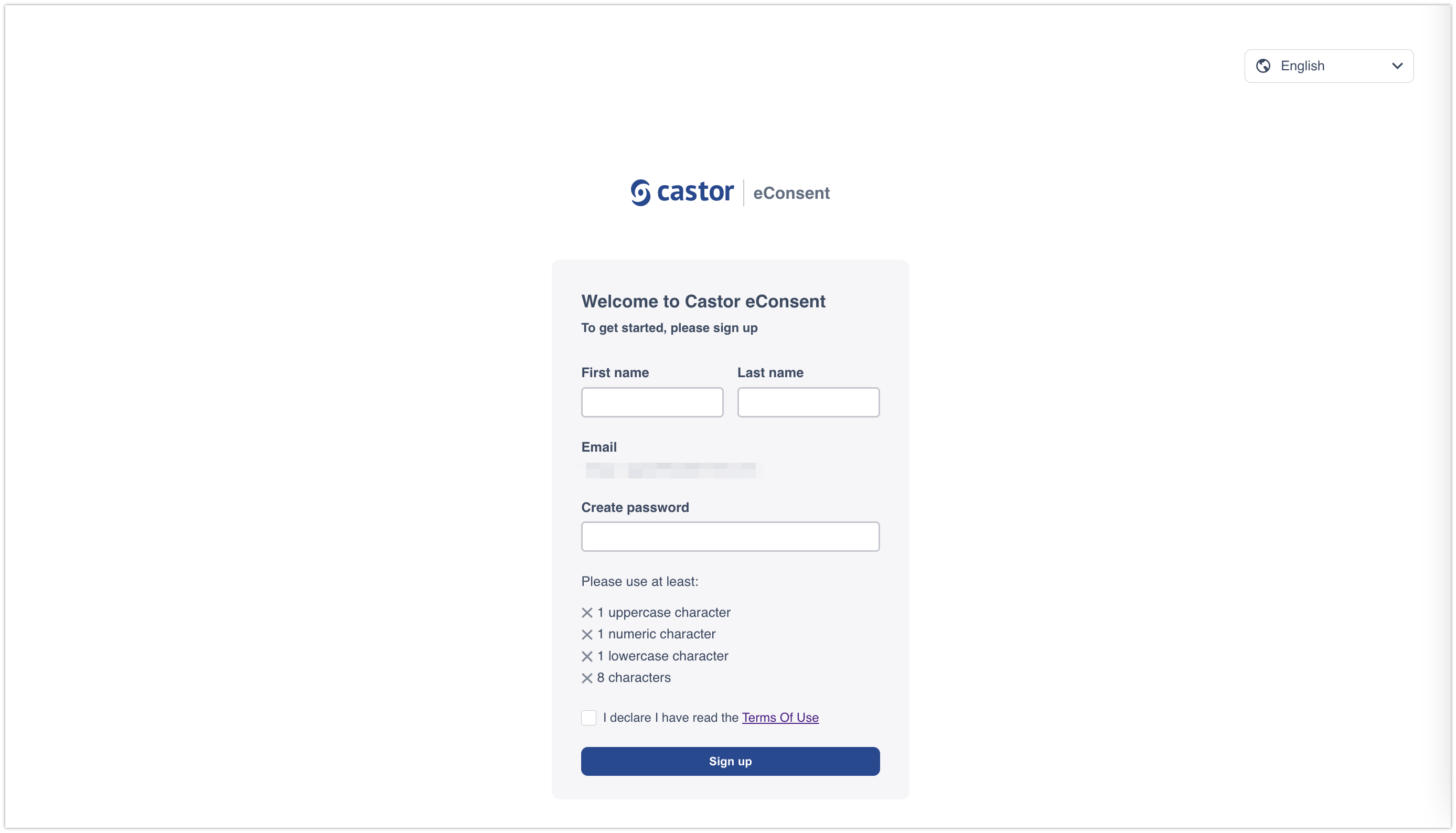
The participant will need to accept the Terms of Use and provide the following details when registering the account:
- First name
- Last name
- Email will be automatically pre-filled
- Create password
After filling out the details and accepting the Terms of Use, the participant needs to click on the ‘Sign up’ button to complete the registration.
Should the participant already have an account registered in eConsent they will be directed to the eConsent login page where they can log in with their eConsent credentials.
During login and signup, participants will be able to select their preferred language for the interface.

Log in
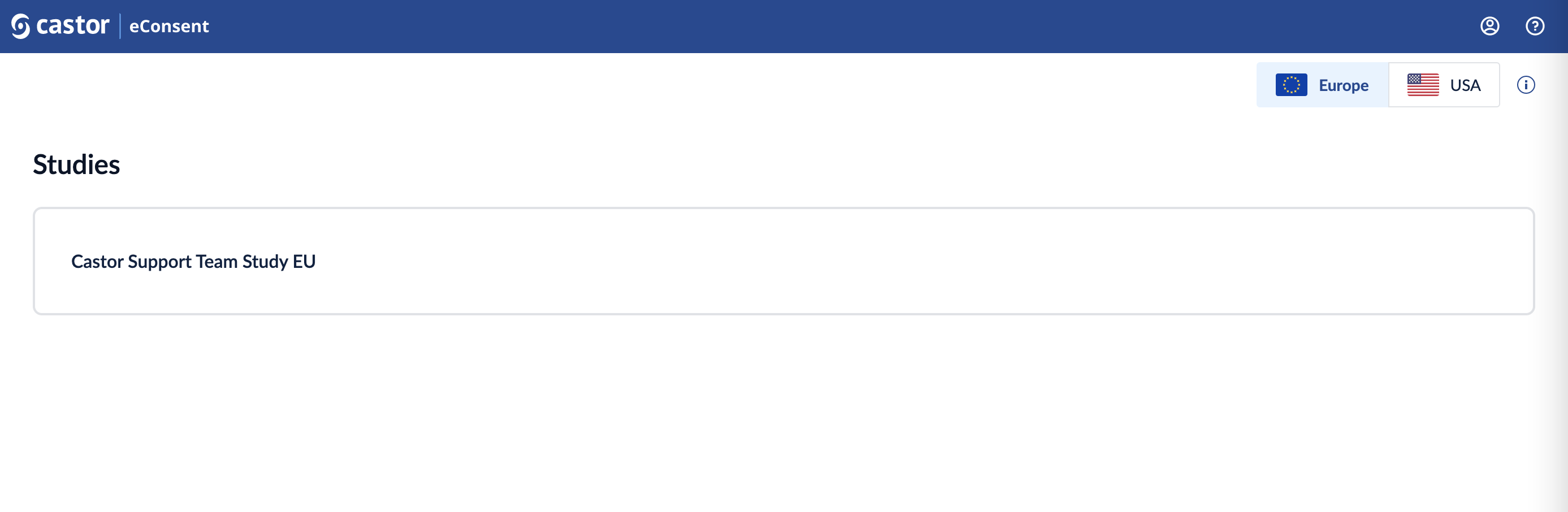
Once registration is completed and the participant has logged in, they can then accept the invitation to the study. Once accepted they will be able to enter the eConsent Study from the ‘Studies’ overview by clicking on the row with the study title.
Dashboard
After accessing a study, participant will be redirected to the Dashboard tab where they will be able to view the study related information and any outstanding tasks for them to complete (for example, Create an account or Sign Informed Consent Form).

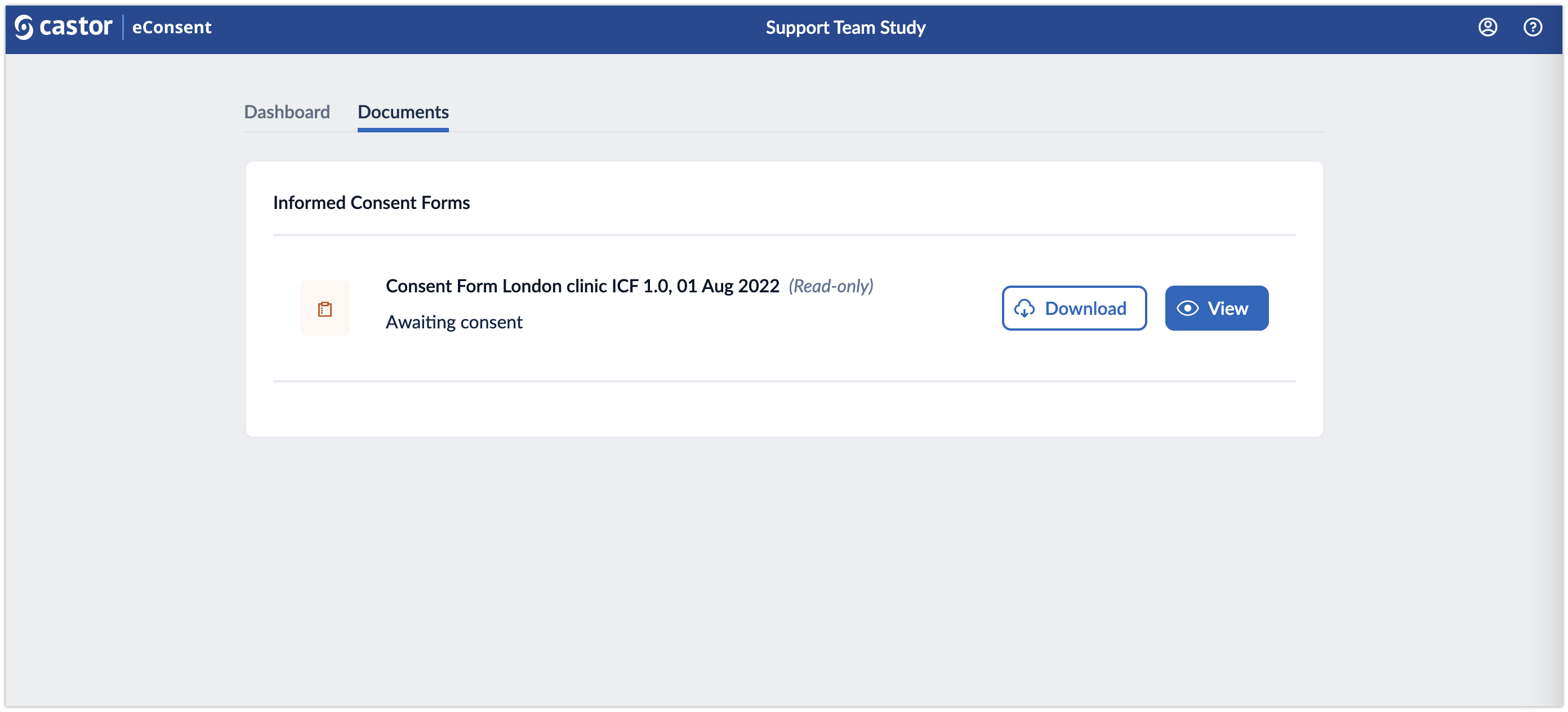
Documents
The Documents tab shows the list of the informed consent forms which are signed or awaiting signature. Participants can choose to download the forms or to view and sign the available forms.
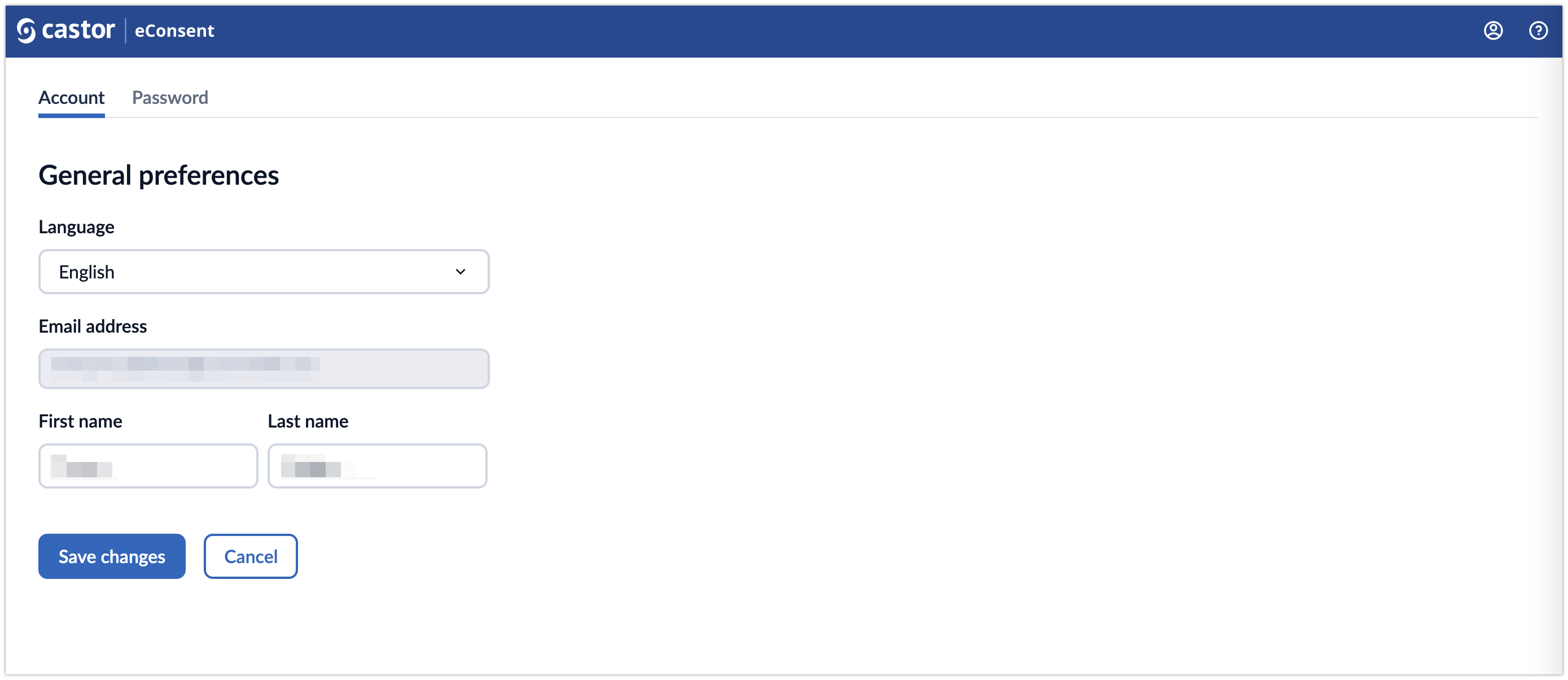
Account Settings
By clicking on the Account icon, participants can manage their account settings.

The Account tab allows to set preferences such as choosing the preferred language, change First and last name.
The Password tab allows to set a new password.