Castor EDC 2021.2 release notes
Table of Contents
Release date: 29 April 2021
Main features & improvements
FormSync
- Creating linked study with duplicated study structure; process can be started from either a test or Real study type and the outcome will be a new study with the same structure but opposite type.
.png)
- ‘Go to study’ option to jump to the linked study added to allow users to easily switch and open one of the other linked studies from the menu.
- Option for unlinking two linked studies added:
- If the user chooses to remove the link/ connection between test and production study this can be accomplished by tapping the ‘Unlink’ button.
- All restrictions that are in place for linked studies (‘study type’ , ‘status; & ‘archive’) are restored to normal after unlinking.

- Necessary rights to use Form Sync:
- Only users with “Manage form” rights can see the “Study link” option from “Structure” tab, otherwise hidden.
- Only users with ‘Manage settings’ rights can create a linked study, review changes, review data impact and apply changes.
- Data impact list added so users can see a summary of all changes that will have data impact on the production study:
- On this view, the system calculates and displays potential data loss & data impact by showing the total number of records that currently exist on the production environment as well as the number of data points and number of records that are affected by each change.
- Reminder/Scheduling as part of Survey Package properties added:
- All properties related to scheduling Survey packages (Send reminder if survey not yet completed:; Reminder subject:; Invitation message:; Send reminder after [x] days:) can be mapped and updated using Form Sync.
- Extension of existing unique identifier fields to allow a correct mapping of the existing Survey Packages between the two linked studies:
- Unique ‘Identifier’ field added to SurveyPackages.
- Unique Variable Name added to Image, Remark, Summary, QR code, RM fields, as well as to Add report and Add survey button.
- On the production (real) study, the SDV, Signature, Custom verifications and Lock status will be automatically dropped.
- Adding a new Option in an Option group without further changes of the order of these AND removing options without any records affected will not cause an automatic drop of verifications.
- Checking for, displaying and updating missing unique identifiers:
- Linking studies AND merging changes ( clicking “Review and merge changes” ) are not permitted if unique identifiers are missing OR not unique.
- Improvement of error messaging to end-user around mapping errors.
-
Study settings:
- The linked test study cannot be set to “live” (disabled function), but editing the ‘Status’ of the linked production study is allowed.
- The type of the 2 linked studies cannot be modified (disabled function for users in StudySettings).
- Changed text for blocked access to structure and forms view:
- As the linked production study structure cannot be edited (disabled function for users), a new informative text has been added on the relevant views.
- Updated label and highlighted linked studies on Studies overview:
- A new icons is displayed in the “My studies” view for all studies that have a linked study.
- On hover-over the icon & text for a study, users can see a dynamic tooltip indicated which is the linked study.
- "Review changes" view added:
- On the production study, users can check to see if changes are available on linked test study.; The changes are presented in a tree view that corresponds to the entire study structure.
- A summary of only the applied changes has been added on in a table view.; Users can switch between the table and the tree view.
- Option to expand/collapse tree elements added, updated labels and display icons on tree view for different types of changes, hiding the search bar on tree and table view.
- Replacement of the current "Version no" with a new "Form Version no":
- In order to see a (production) study form ‘version’ that corresponds to the number of previously applied changes from test to prod (how many times changes were pushed from test to prod), we added a new Form Sync version number that increases every time a successful sync is performed.
- Option to export full study structure and data before applying changes from test:
- In order to be able to export a complete backup, only users with full rights enabled across all institutes (and not just export rights across all institutes) can proceed to download the backup.
- Audit trail specifics for form sync added to keep track of changes and comply to regulations:
- When 2 studies are linked or unlinked, new events ("Linked study created" & "Study unlinked") are created and added to the audit trails of both the test and the production study
- When changes are pushed from the test study to its linked production study, new events ("Sync from source study started" & " Sync from source study completed" are created and added to the audit trail of the production study
- Unique identifiers autofilled and enforced to have all of them filled for the linked studies.
- Images stored in the image field can be edited and reflected in both linked studies.
- To avoid further data loss/ data impact, field type changes are restricted to only allow compatible ones.
Surveys
- Russian language to the patient-facing UI is added to Surveys

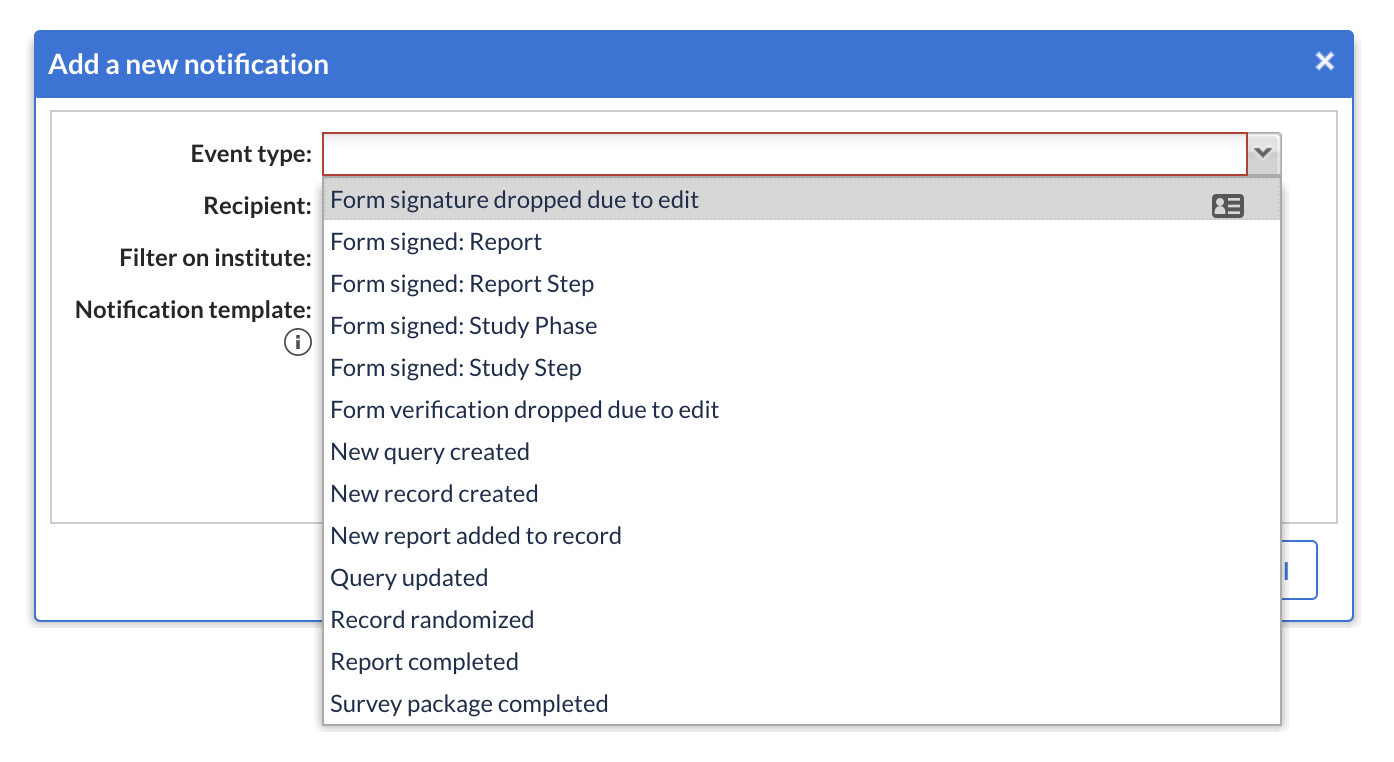
Notifications
Study settings notification events are added in Notifications in the Settings tab:
- For ‘Form signed’, four new notifications are created and added: Study Phase, Study step, Report step, Report and a secondary dropdown appears according to the selection.
- For ‘Report completed’, secondary dropdown ‘Select report’ appears.
Monitoring: Verifications
In ‘Verifications’, dropping field-level SDV events are now displayed at both record-level (Data Entry) and for all records (Monitoring tab).
- If field-level SDV is dropped due to changes to that field (edit value/ ‘user missing’), the event is logged and displayed in the Verifications tab.
- If field-level SDV is dropped due to Record(s) import, the event is logged and displayed in the Verifications tab.
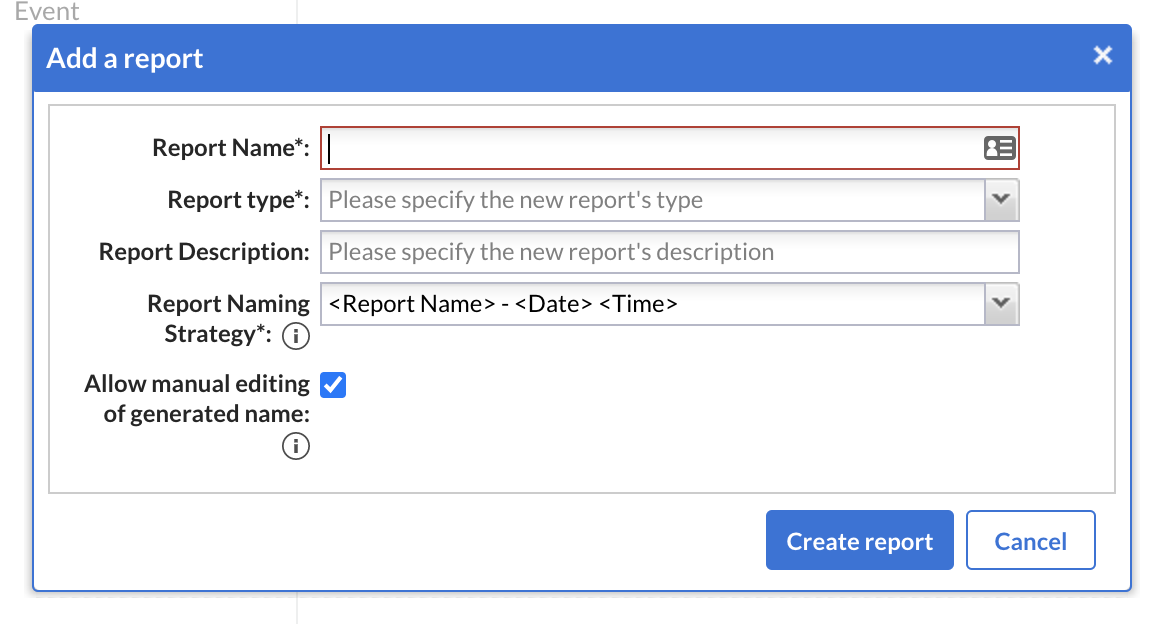
Report naming strategies
- Report naming strategies were implemented where a user can select from multiple options on how report names can be generated.
- ‘Report Naming Strategies” can be configured on the Report level.
- The report name will be generated upon creating each report instance and based on your selected Report naming strategy.
- The user can allow/disallow manual editing of the generated name.
Data entry
Improved handling of exclusions (navigation between records, banners, label colors, positions, copy):
- Navigation between steps & phases is allowed on excluded records.
- if an exclusion occurs on the study form, data entry is blocked on the entire study form & on any report instances.
- if an exclusion occurs on a report instance form, data entry is blocked on that report instance form but not blocked on any other report instances or study data.
API
Memory-optimize the /api/export/data endpoint (user exporting data with export rights, not able to export encryption and randomization field data through api...)
Castor Connect
Foundational work implemented in Castor EDC to enable the coming release of our native app for ePRO/eCOA called Castor Connect.
Defect fixes & minor improvements
- Institutes now can be updated to be ‘Main institutes’ in the study settings.
- Email addresses are no longer case sensitive upon signing forms.
- Reports can be printed now if there is a slider with a user missing value.
- Dropdown values do not disappear after adding comments.
- When creating a new study, the first field “Name” is now focused so that a user can directly start typing.
- Data entry is not possible anymore for user roles with exclusion message triggered in blinded step.
- Validation messages, option groups, radio buttons and checkboxes remain visible after adding comments.
- Floating data points are no longer imported with extra decimals.
- Record instances show history of their own record, not other record instances.
- No error messages are logged when marking a phase as missing that has AE-hidden steps.
- Validation messages no longer remain in the monitoring tab after hard-deleting survey invitations.
- Option groups are no longer replaced with existing option groups with the same values upon form structure import.
- User no longer has to refresh the form after clearing the source fields.
- Sliders and grid fields get disabled when a field value triggers an exclusion.
- Copying a survey with an image now also copies the image.
- Grid field cogwheel in survey data entry is now clickable and working.
- Warning message is added when user applies 'SDV all fields' to a step with only non-required fields.
- Updated warning message is now shown for Reports that are hidden for certain roles.
2021.2.1 - 30 Apr 2021
- Fixed a bug where some dropdowns displaying all phases of the study required manage form rights, removed the requirement for the rights now.
- Fixed a bug where creating a form sync study would time out after 30 seconds.
2021.2.2 - 30 Apr 2021
- Fixed a bug where user missing values for number fields were not imported successfully.
- Fixed a bug where users could not delete a survey schedule.
2021.2.3 - 7 May 2021
- Fixed a bug where scheduled surveys were not sent.
- Fixed a bug where exports failed for studies with export optimization enabled.
- Added 'archived' property for all API report endpoints.
- Improved unclear error message when locking a form in locked record.
- Security fixes on user rights and survey groups
- Fixed a bug where users without edit rights could not click the reports in a repeated measures grid.
- Improved the performance of retrieving survey package instances for studies with lots of survey package instances.
- Fixed a bug where updating report instance names was not working.
- Fixed a bug in the automation engine where the phase dropdown would not load any phases.
- Fixed a bug where adding a report instance from a repeated measures grid did not open the report instance, but directed the user to the reports overview.
2021.2.4 - 14 May 2021
- Fixed a bug where an add report button would create a new report instance, but did not open the form.
- Adjusted the font on the register page.
- Fixed a bug where form sync would break when a field was changed from slider to a text field, and the minx/max options were cleared.
- Fixed a bug where form sync would timeout for specific studies.
- Security fix on Query remarks dialog.
- Fixed a bug where generating a new record displayed a blank page in IE11.