Sign and lock a completed participant at once in CDMS
Instead of signing and locking the Forms/Visits one by one, users with ''Sign'' rights can now directly sign off all visible (according to the user's role) forms, visits, repeating data instances of a single participant from the Participants overview.
To perform this, click on the 3 dots next to the participant and click on the ''Apply signature option''.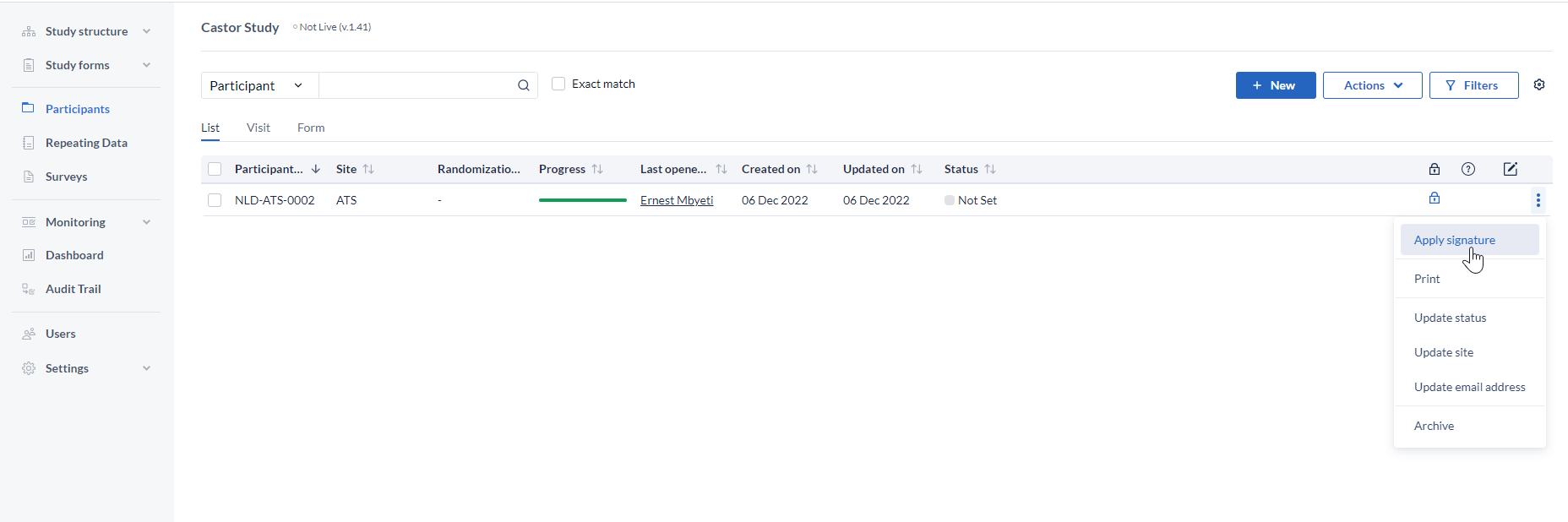
You will be prompted to enter your credentials in order to sign the participant. Additionally you can also select to lock the participant upon signing, by ticking the ''Lock participant'' box.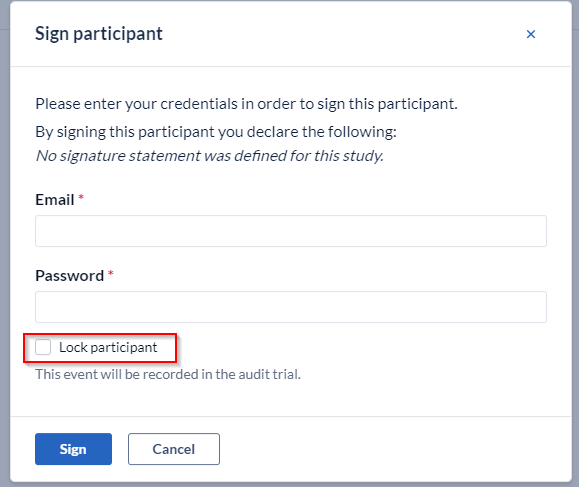
Once the signature has been applied all the forms, visits and repeating data in the participant will automatically be signed too.
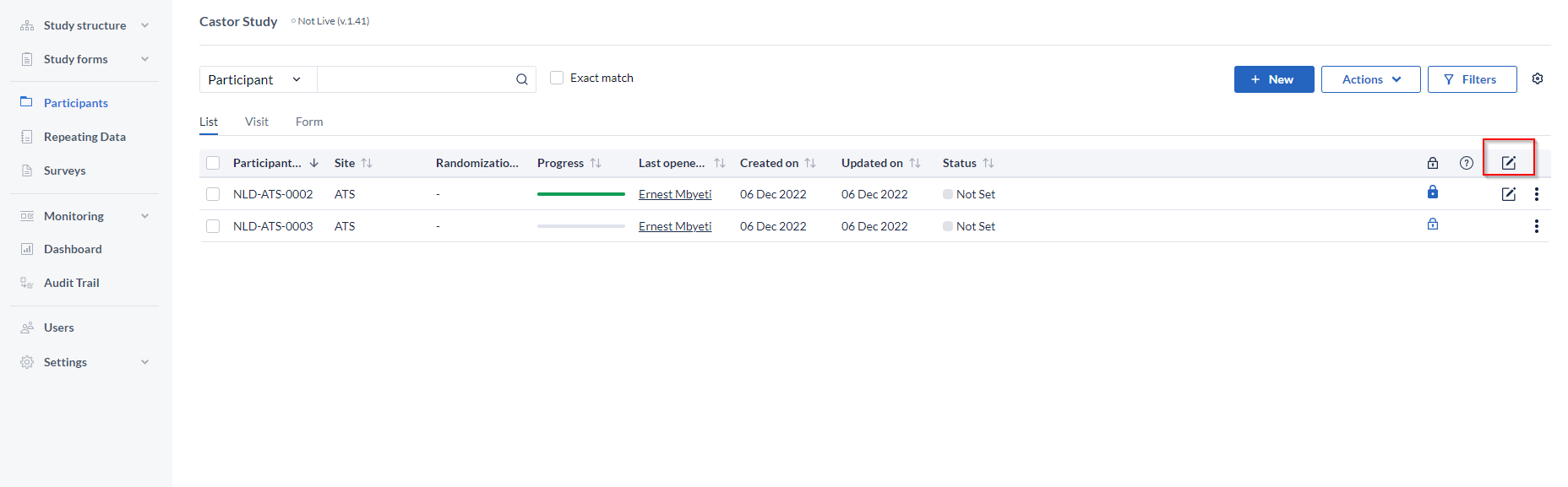
A new column is available on the participants overview to indicate the signature status of a single participant. The icon will be displayed only when all forms and visits of the participant have been signed.
By default the column is selected, but it can be removed from the view by unchecking it from the cogwheel menu.
Once the signature has been applied all the forms, visits and repeating data in the participant will automatically be signed too.
NOTE: If a user has been blinded from seeing a form or visit of the study due to the user roles, the option to sign will still be available but the form or visit that is hidden from them will not be signed and the signature icon will not be shown. When this is the case the user will be shown the below message: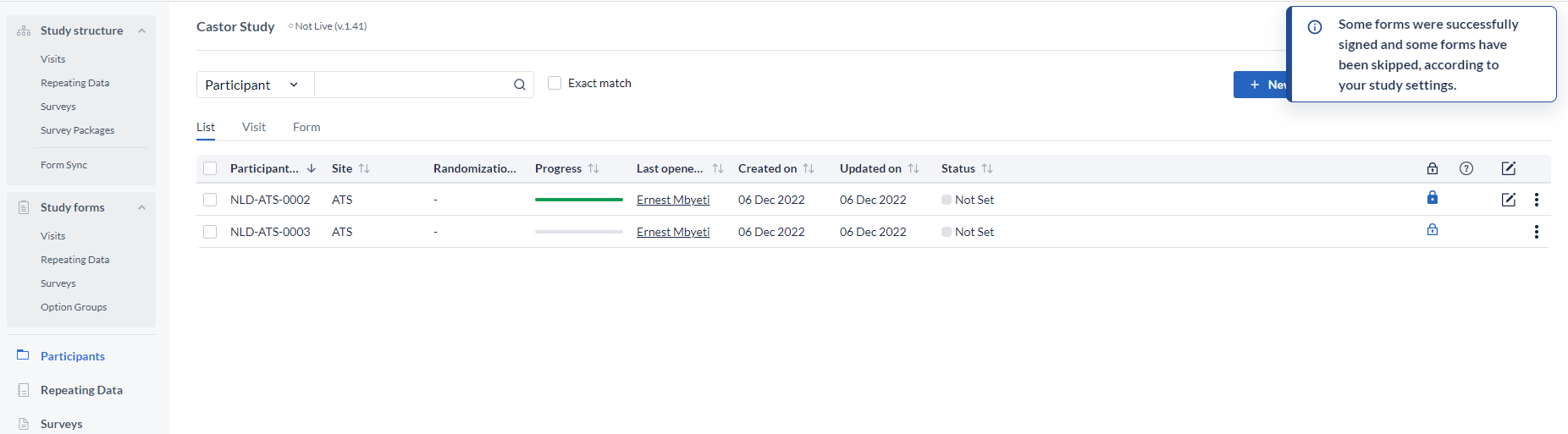
Additional information
- The signature of the participant will drop if any structural changes are performed. For example adding a field or repeating data instance to a signed participant will cause an automatic drop of the signature.
- If a signed participant is archived, the signatures will not drop and upon unarchiving it it will remain as signed.
- When you manually sign/unsign a participant the archived repeating data instances do not get affected. When you sign/unsign a participant using other scenarios e.g. structural changes, the archived repeating data instances are also affected and logged in the Audit Trail.
- When a repeating data instances is unarchived, the sign logic is recomputed and updated automatically and any relevant Audit Trail events are logged in the Audit Trail.
- Finally these events can be tracked through the audit trail by using the events ‘’Participant signed'' and ‘’Participant signature dropped''. These events are captured both when they are triggered manually from a user and when they are triggered automatically as a consequence of other changes (for example, adding a new form/visit to a signed participant).
- It is worth noting that if a signature is dropped automatically as a consequence of another change, for example changing data in a signed participant, the audit trail will capture this drop as having been done by ‘’System user'' and will not contain any additional information such as the signature statement.