Linking two studies in CDMS
Table of Contents
Before attempting to link two studies, make sure that you have the necessary user rights. Only users with “Manage settings” rights can create a linked study, review changes, review data impact and apply changes (otherwise all actionable buttons are disabled).
Option Groups
Option Groups that are not being actively used in your study forms may be displayed as deletions and / or additions on the overview of changes, as they are not part of the study’s structure. We recommend maintaining only Option Groups that are used in your study for a seamless experience while applying mid study changes.
Unique identifiers
Form Sync uses identifiers to map elements between the two linked studies. These identifiers are unique across the entire study, automatically generated by the system and stored in the backend. The unique identifiers are available for the following elements: Option Groups, Surveys & Survey forms, Repeating Data & Repeating Data forms, Options, Validations, Fields.
Linking two studies
When creating a new study, it is possible to choose between study types Real, Test and Example. Choosing the Real study type will set the current study to be Production study automatically.
Choosing Test study type will set the current study to be the Test study by default. You will be prompted to create a Linked Production study in this case. The Form Sync option is not available if an Example study type is chosen. You can change your study to another study type (Real or Test) in the Settings tab to enable the Form Sync option for your study.
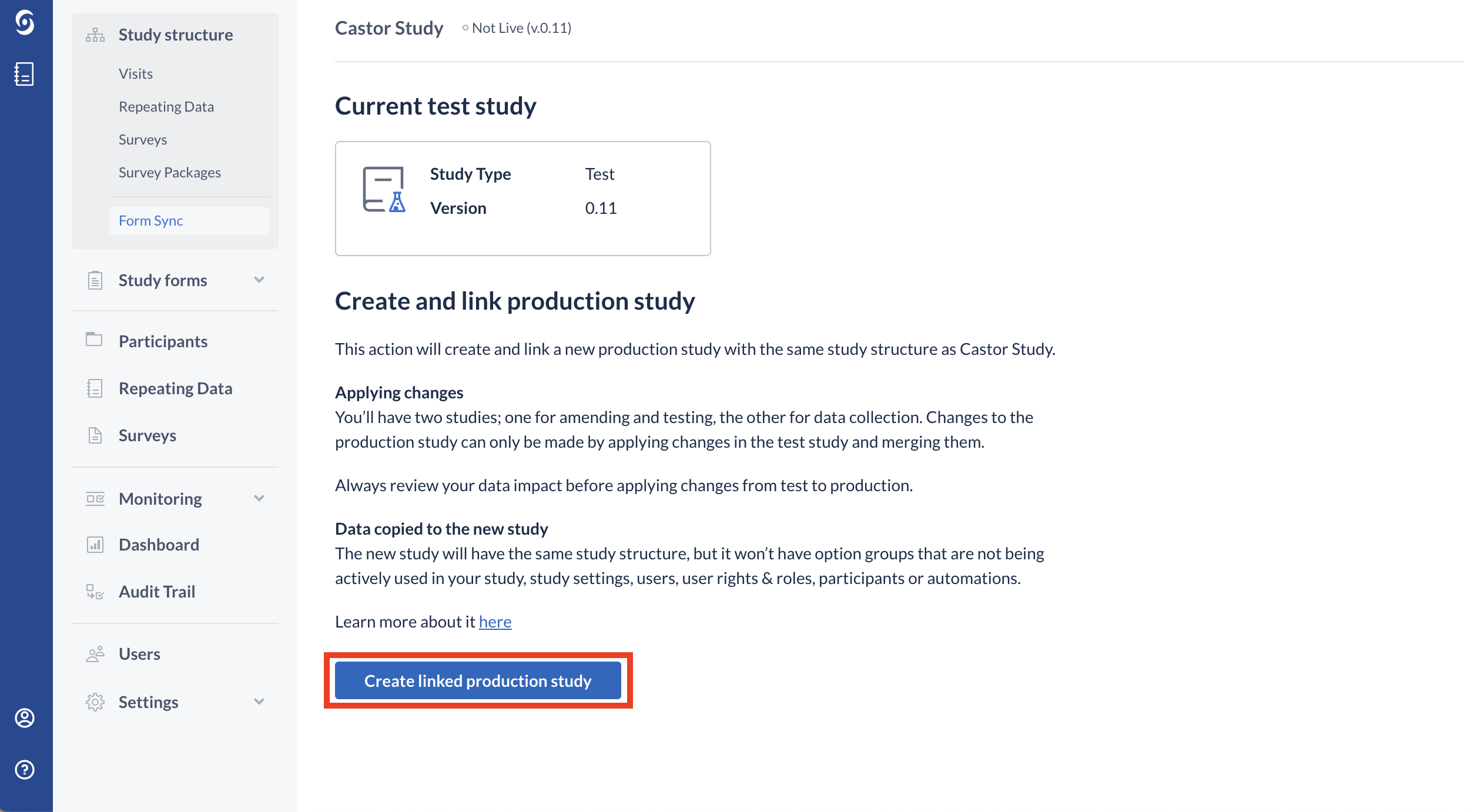
To link two studies, follow the steps below:
1. Click on the ‘Create Linked Test Study’ or ‘Create Linked Production Study’ button depending on the study type.
2. Once the two studies are linked together, new icons are displayed in the “My studies” view for all studies that have a linked study. While hovering-over the icon and text for a study, users can see a dynamic tooltip indicating which is the linked study.
Please note the following:
- Creating an initial linked study might take a while for studies with many visits and forms.
- No other properties of the source study will be automatically copied, such as further study settings, automations, randomization, users, user rights and roles.
- The name of the newly created test or production study is automatically generated: it contains two parts linked by an underscore “_”: the study type and the name of the source study.