Changing the study administrator in CDMS
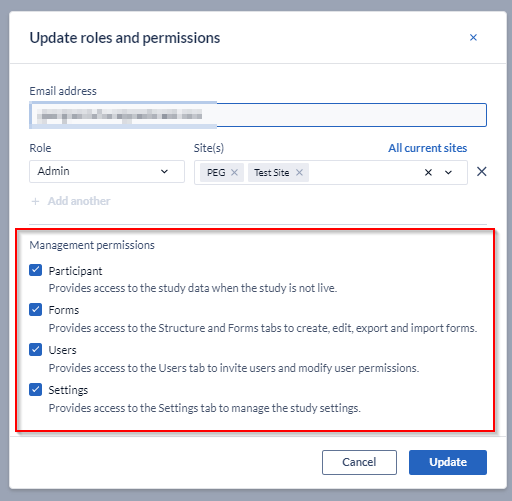
To hand study ownership to another user, you should first update the user rights to ensure their access level is equivalent to the study admin. Normally this requires granting all the Management rights.
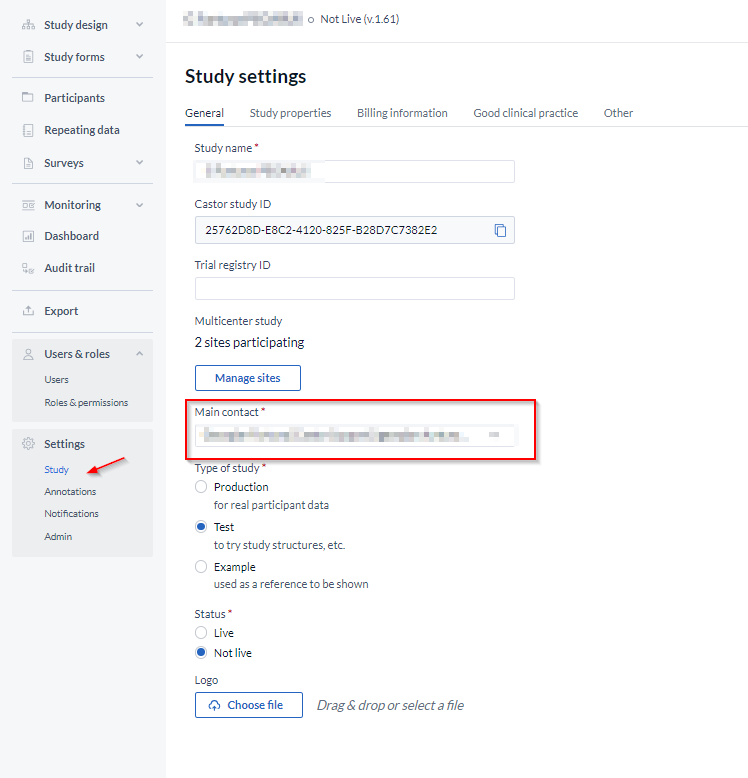
Once all user rights have been updated, you can edit the 'main contact' information in the Settings tab.
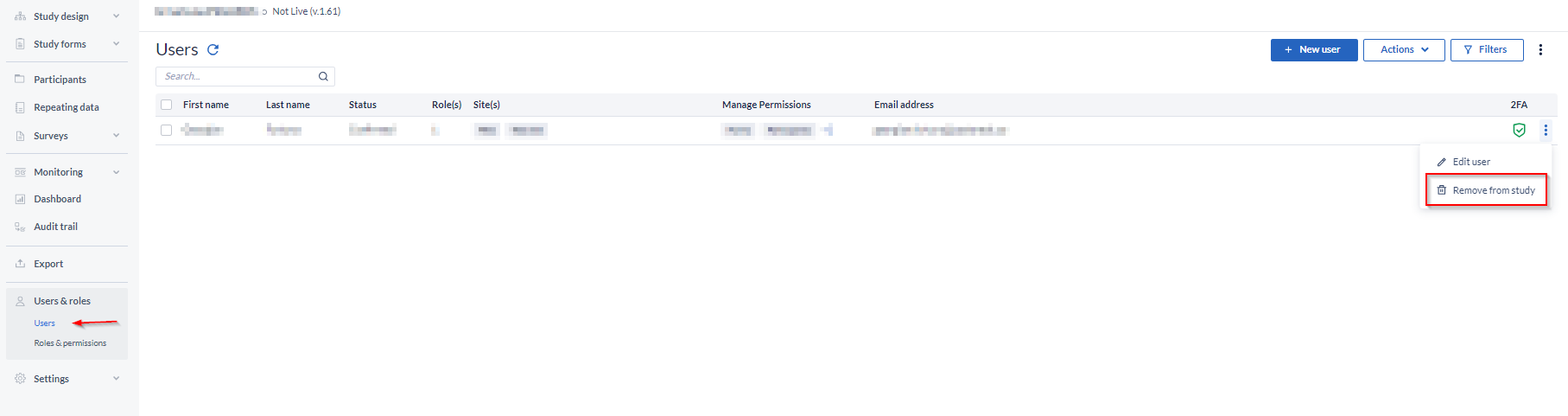
If you no longer intend to participate in the study, you can remove all your user rights and ask the new study admin to remove you from the user's list or just simply delete yourself from the study.
If the study has the “Field encryption” feature enabled on the study Settings the admin will also have the possibility to assign Encryption user roles, per site.
More on this can be found in the following article: